How to Identify and Classify Amines
Examples and Characteristics
Chemical Reactions
Essentially, amines are aliphatic or aromatic ammonia derivatives where one or several hydrogen atoms are replaced by a carbon group (alkyl/aryl group). Like ammonia, amines are weak bases and therefore do not fully ionize in an aqueous solution.
In nature, amines can be found in proteins, alkaloids, vitamins, and hormones where they occur naturally. However, they can also be found in a number of synthetic compounds including drugs and dyestuffs among others.
* While there are different types of amines, they are all characterized by the presence of a nitrogen atom.
Identification of Amines
As mentioned, there are different types of amines which are classified under four distinct groups. Before looking at the different types of amines and associated characteristics/properties, this section will focus on some of the characteristics shared by all or the majority of amines for identification purposes.
Odor - In general, amines have a pungent/noxious odor that makes it possible to identify them. Like ammonia (which is often regarded as the simplest amine), amines with a low molecular weight have a strong odor that tends to be irritating.
Good examples of amines with an ammonia smell include methylamines and ethylamines which are some of the simplest amines in nature. Higher amines (amines with a higher molecular weight), on the other hand, are characterized by a fish-like smell. As such, they are responsible for the rotting, fish-like smell associated with decaying tissue.
In rotting fish, trimethyamine is responsible for the fish-like smell. The decomposition of given amino acids (e.g. arginine and acid lysine) in rotting flesh results in the production of such amines like putrescine (1,4-diaminobutane) and cadaverine (1,5-diaminopentane) which are responsible for the bad, fish-like smell.
Basicity - Generally, amines are referred to as Lewis bases because of the fact that they can donate an electron pair. It's worth noting that basicity varies between different types of amines depending on the properties of the substituents of the amine, level of solvation (reorganization of solvent and solute molecules) as well as steric hindrances.
Regardless, all amines are basic and are therefore capable of sharing atoms. Because all amines have an unshared pair of electrons (like ammonia), they have been shown to share chemical behavior with ammonia.
Solubility - Generally, amines are more soluble in dilute acids than they are in water. Solubility of amines in water varies between different types of amines. In acids, the majority of amines react to forms salts that can then dissolve in water.
While octylamine is insoluble in water, it can react with nitric acid to form octylammonium nitrate which is soluble in water. Therefore, one of the methods that can be used to test whether a given compound is an amine may involve reacting it with an acid (e.g. HCL) which converts it into a salt that can then dissolve in water.
Boiling point - Generally, amines have a higher boiling point when compared to various hydrocarbons but lower compared to alcohols. As such, some of them tend to be gaseous at room temperature (e.g. methylamine and trimethylamine) while liquids are easily vaporized.
It's worth noting that some of the amines, particularly the ones with a higher molecular weight, are solids at room temperature (e.g. tripropylamine).
Classification
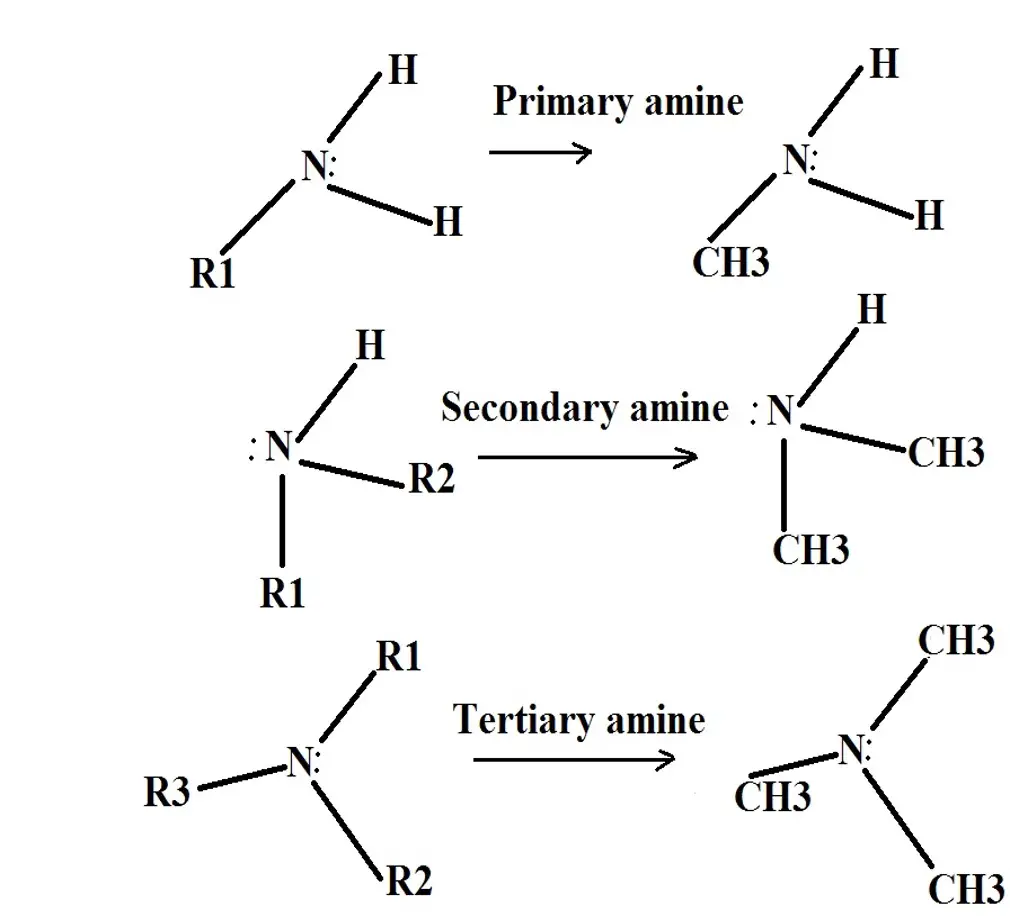
In general, amines are divided into 4 major classes/types that include; primary, secondary, and tertiary amines.
Primary Amines
Primary amines can be described as a derivative of ammonia where a hydrogen atom is substituted with an alkyl or aryl group. In this case, then, only one group, alkyl or aryl that replaced the hydrogen atom, is attached to the nitrogen.
A good example of a primary amine is methylamine which has the following chemical formula: CH3-NH2 - Generally, the chemical formula of primary is presented as RNH2, where R represents the alkyl or aryl group and the N (nitrogen), has a pair of free electrons.
One of the main characteristics of primary amines is that they tend to be less volatile compared to hydrocarbons (those with similar weight, size, and shape). This characteristic is attributed to weak hydrogen bonding present in primary amines (N-H....:N). The primary bond (between carbon and nitrogen) is primary amines is also relatively smaller compared to such alkanes as ethane.
Whereas the carbon-nitrogen bond in methylamine has been shown to be 1.47 angstrom, the carbon-carbon bond in ethane is 1.53 angstrom. This is because of the fact that compared to the carbon-carbon bond in ethane (non-polar), primary amines are slightly polar covalent where the nitrogen atom pulls electrons stronger as compared to the carbon in ethane.
As well, the angles between the hydrogen and R groups are also different compared to the angles found in ammonia. Whereas the angles between hydrogen atoms in ammonia are 107.5 degrees, the angle between the hydrogen atoms in a primary is 106 degrees while the angle between a hydrogen atom and the R group is 112 degrees.
This is due to the fact that the R group is a larger group that causes a larger electrostatic repulsion that pushes the hydrogen atom slightly more than is the case in ammonia.
Secondary Amines
In a secondary amine, two hydrogen atoms are replaced by alkyl/aryl groups. This means that the compound consists of two alkyl/aryl groups and a single hydrogen atom. Therefore, the following chemical formula is used to represent secondary amines: R2NH.
Here, the R group may be the same as is the case with dimethylamine, which consists of 2 CH3, and diethylamine (which consists of 2 CH2CH3).
Like primary amines, secondary amines are also weaker acids and tend to form strongly basic anions when compared to alcohols. In addition, they have also been shown to be less volatile when compared to corresponding hydrocarbons (those with the same weight, size, and shape).
Compared to corresponding primary amines that have the same number of carbon atoms, however, secondary amines have a relatively lower boiling point which is one of the characteristics used to differentiate between the two. The lower boiling point is a result of the lower dipole-dipole attractions in the compounds.
Tertiary Amines
Unlike primary and secondary amines, tertiary amines do not have any hydrogen atoms. This is because all the hydrogen atoms of the ammonia molecules are replaced by R groups.
The following formula is used to represent tertiary amines: R3N (where the nitrogen contains a free pair of electrons). A good example of tertiary amine is trimethylamine which consists of three methyl groups.
Due to the presence of hydrogen atoms in primary and secondary amines, these are characterized by intermolecular associations resulting from the bonding between the nitrogen of one molecule and hydrogen or another molecule. Given that tertiary amines do not have any hydrogen atom, this type of association is not present in these molecules.
Unlike the other two amines that have hydrogen atoms, tertiary amines have also been shown to have a lower boiling point. On the other hand, they tend to react slower when compared to secondary amines.
Because they lack hydrogen atoms, the angles between the groups are also much smaller when compared to those in primary and secondary amines (the angle between hydrogen and the R group (s)). Here, the angles between the groups have been shown to be 108.4 degrees.
As mentioned, this is due to the relatively stronger electrostatic repulsion between the R groups.
* There is a fourth group of amines known as Quaternary Amine. As the name suggests, these amines have four R groups bound to a nitrogen atom. They do not have any hydrogen atoms.
The nitrogen of these amines has a net positive charge. Also known as Quaternary ammonium cation, these amines are often produced through the alkylation of tertiary amines
Chemical Reactions to Distinguish between Primary, Secondary, and Tertiary Amines
As mentioned, the three types of amines share a number of characteristics that qualify them as amines. They also have several differences (e.g. boiling point and structural characteristics, etc) that make it possible to differentiate between them. In addition to these characteristics/properties, there are several tests that can be used to identify them.
These include:
Hinsberg Test
The Hinsberg test is one of the most commonly used tests for differentiating between the three types of amines, primary, secondary, and tertiary. The amine of interest is mixed with the Hinsberg reagent and the results analyzed. For the different types of amines, results will be different indicating the presence of given amines.
Procedure:
Basically, the Hinsberg test follows the following steps:
· 8 to 10 drops of the amine to be tested are first added into a test tube
· 10 drops of benzyme sulfonyl chloride are then introduced into the tube followed by 10ml of 10 percent sodium hydroxide
· The contents are shaken to mix
Results:
· If a single layer of the mixture is produced, this indicates that the amine is a primary amine
· If two layers are produced in the solution, this indicates that the amine was a secondary amine
· A solid or oil product that dissolves in hydrogen chloride to form a clear solution indicates that the amine is a tertiary amine
Ramini Test
Unlike the Hinsberg test, a Ramini test is commonly used to differentiate between primary and secondary aliphatic amines (amines where aromatic rings are not directly attached to the nitrogen atom).
Here, the test involves reacting an amine with acetone before introducing the product to sodium nitroprusside in 50 percent aqueous methanolic solution. In the event that the result is a red coloration, this is indicative of primary amines.
Simon Test
The Simon test is commonly used to determine whether secondary amines are present and thus distinguish them from primary and tertiary amines. This test is similar to the Ramini test except for the fact that acetone is replaced with acetaldehyde solution. If a blue-green coloration is produced after two (2) minutes, then the amines are secondary amines.
Nitrous Acid Test
This test is used to distinguish between the three types of amines as well as between aliphatic and aromatic amines.
Results of this test include:
· Formation of a diazonium salt - For the production of an intermediate diazonium salt is indicative of primary aromatic and aliphatic amines
· Diazonium salts decomposition - indicates the presence of aliphatic amines. As well, the diazonium salts of aromatic amines remain stable at 0 degrees C
· Yellow oils/solids - indicates the presence of secondary amines
· Soluble salts - indicates the presence of tertiary aliphatic amines
· Orange coloration - indicates the presence of tertiary aromatic amines
Nomenclature
Naming amines is an important process that allows different amine compounds to be identified based on their constituents. In order to name a given amine compound, several steps have to be taken into consideration. However, before taking any step, it's important to first determine whether the functional group, NH, is present.
It's worth noting that this functional group may be present in the form of NH, NH1, or NH2 where "N" represents a nitrogen atom and "H" represents the hydrogen atom. The presence of the functional group means that it is an amine.
Step 1: Identify the longest chain with a carbon that is attached or contains the functional group (amine).
The following is a good example of step 1:
CH3CH2CH2-NH-CH3
Looking at this compound, it's evident that the longest chain with a carbon that contains the functional group is CH3CH2CH2 (propan). Being the longest chain, this group makes up the parent chain of the compound. Because this chain contains the functional group, it becomes propanamine.
Step 2: Number of carbons
The second step involves counting the number of carbons in the longest chain with the carbon that contains the functional group.
Counting the number of carbons should always start with the carbon close to that which contains the amine/functional group. This is particularly important given that it allows for the identification of a carbon that may contain an additional constituent. If another constituent is not present, then proceed to step 3.
Step 3: Identify any other substituent attached to the functional group
In our example, the only other constituent that is attached to the functional group is a methyl (CH3). Once it's confirmed that this is the only other constituent and that it's attached to the functional group, then we can proceed to step 4.
Step 4: Name the amine
By the time we get to step 4, all the constituents of the compound have been identified. Therefore, we can now name the amine. As mentioned in step 3, we identified that the methyl is the only other constituent that is attached to the functional group.
As such, we name it N-methyl given that the carbon on the methyl is bound to the nitrogen of the functional group. Now that all the components are named, we can combine the names in order to fully name the amine. Here, we start with the N-methyl so that we eventually have: N-methyl-1-propanamine.
* In step 2, we counted the number of carbons from the carbon close to the carbon bound to functional group. Given that carbon 1, in this case, is attached to the functional group, then we add a "1" in front of the propanamine to indicate that our functional group is attached to the first carbon of the longest chain.
In the event that the functional group was attached to another carbon, then the appropriate number (of the carbon) would have to be used.
Return from How to Identify and Classify Amines to MicroscopeMaster home
References
Kevin A. Boudreaux. Amines and Amides.
Stephen A. Lawrence. (2004). Amines: Synthesis, Properties and Applications.
Večeřa M., Gasparič J. (1971) Amines. In: Detection and Identification of Organic Compounds. Springer, Boston, MA.
Links
https://www.chemistrylearner.com/hinsberg-test.html
https://www.chemguide.co.uk/organicprops/amines/background.html
Find out how to advertise on MicroscopeMaster!