Endothelial Progenitor Cells (EPCs)
Markers, Isolation and Angiogenesis
Engineered Tissues and Cardiovascular Disease
Overview
As the name suggests, “endothelial progenitor cells (EPCs) are progenitors with the ability to produce functional endothelial cells." Based on recent findings, endothelial progenitor cells have been shown to be heterogeneous and are associated with several other functions.
Originating from the mesodermal stem cells, these cells are commonly known as angioblasts in embryos (embryonic endothelial progenitors). In adults, they are divided into two main groups that which include endothelial progenitor cells of hematopoietic origin (known as myeloid angiogenic cells) and those of non-hematopoietic origin (endothelial colony-forming cell).
A portion of these progenitors are suggested to reside in various tissues (e.g. the neural tissue and adipose tissue, etc) and are referred to as tissue-resident endothelial progenitors.
Some of the main characteristics of endothelial progenitor cells include:
- Capable of self-renewal
- Can differentiate to give rise to functional cells
Markers
Markers are important molecules that make it possible to differentiate different types of cells. Consisting of proteins or lipids etc, the majority of these molecules are associated with the cell membrane and are therefore described as cell surface antigens in many works of literature.
Like many other cells, endothelial progenitors are characterized by several types of markers, these include:
CD133 -Also known as prominin-1, CD133 is a membrane glycoprotein that can be found in human beings and mice.
CD34 - Encoded by CD34 genes in human beings, CD34 is a transmembrane phosphoglycoprotein (sialomucin) found in human beings
Vascular endothelial growth factor receptor-2 (VEGFR2/KDR/Flk-1) - Expressed on the surface of endothelial progenitors and some endothelial cells, vascular endothelial growth factor receptor 2 is a receptor (tyrosine kinase) that has been shown to moderate the angiogenic activities of VEGF-A (Vascular endothelial growth factor A) and VEGF-C (Vascular endothelial growth factor C)
VE-Cadherin/CD144/Cadherin-5 - Vascular endothelial cadherin is encoded by the CDH5 gene in human beings and belongs to a subgroup of Cadherin homophilic adhesion proteins. As such, it's involved in adhesion and can therefore be found between endothelial cells
CD31 - Like VE-Cadherin, CD31 (PECAM-1) is also an adhesion protein. It's encoded by the gene PECAM1 (Platelet endothelial cell adhesion molecule) and is heavily glycosylated
CD45 - Encoded by the PTPRC gene, CD45 is a transmembrane protein that is also known as protein tyrosine phosphatase receptor type C (PTPRC)
CD177 - CD177 is a glycosylphosphatidylinositol-anchored protein that is encoded by the CD177 gene. It's also known as c-kit and acts as a cellular receptor
CXCR4 (C-X-C chemokine receptor type 4) - Also known as leukocyte-derived seven-transmembrane domain receptor (LESTR), CXCR4 is a G protein-coupled receptor that is encoded by the CXCR4 gene
Some of the other markers associated with endothelial progenitor cells include:
- ETV2/ER71 - involved in hematopoietic regeneration
- Tie-2 - cell surface receptor
- CD146 (MCAM) - adhesion molecule
- VEGFR3 - member of class III receptor tyrosine kinases
Isolation
Endothelial cells can be isolated from a number of sources in the body. Whereas some of these cells can be found in the peripheral blood and umbilical cord blood (non-hematopoietic), others can be found in neural tissue, umbilical cord tissue, as well as adipose tissue, etc (tissue-resident EPCs). Therefore, endothelial progenitor cells can be isolated from several sources.
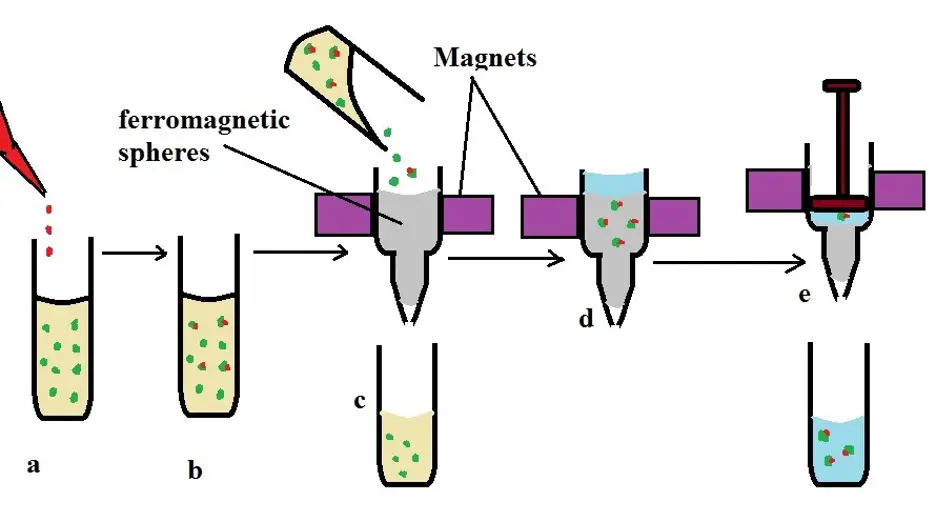
While there are a number of techniques that can be used to isolate these cells, magnetic microbeads coated with the appropriate antibodies are commonly used for this purpose.
In general, using magnetic microbeads (column-based magnetic cell separation) for isolation may involve the following steps:
· Sample collection - If peripheral blood is being used, then a sample is first collected - The sample has to be properly stored if it's not used immediately
· Labeling - The second step of isolation involves labeling the cells with microbeads for a few minutes at 4 degrees C. In this case, the microbeads (magnetic) can be coated with anti-CD133 or anti-CD34 antibodies so that they can combine with the antigens on the surface of the progenitors
· After a few minutes, the cell suspension is passed through a column matrix which is placed in a magnetic field. While the unlabeled cells easily pass through, the magnetically labeled cells are retained in the column
· By pushing buffer through the column, it becomes possible to collect the labeled/target cells which can then be washed and assessed
a) Cells are labeled with antibody-coated microbeads
b) labeled cells (green and red) and unlabelled cells (green)
c) both labeled and unlabelled cells are passed through a column located between magnets
d) labeled cells are retained in the column - buffer is also added into the column with labeled cells
e) the buffer is pushed out of the column to collect labeled cells
Angiogenesis
Angiogenesis refers to the process through which blood vessels develop from the existing vascular system (vasculature). The process occurs throughout the life of an individual from fetal development to old age.
In postnatal life, this process is involved in a number of other roles including tissue ischemia, wound healing, as well as the formation of tissue vasculature.
In an embryo, studies in mice have shown that angioblasts (endothelial progenitor cells in embryos) originate from the mesoderm cells, which themselves are produced in the posterior primitive streak. From the mesoderm cells, these precursors migrate to the extraembryonic yolk sac where they are involved in the formation of the primitive capillary plexus.
As more of the angioblasts are produced from the mesoderm cells, they migrate to the embryo proper where they continue forming the systemic vascular capillary bed. Following the formation of these initial capillaries, expansion of the vessels occurs through angiogenesis and vasculogenesis.
With regards to angiogenesis, some of the factors that induce this process include:
- Platelet-derived growth factor
- Transforming growth factor beta-1
- Epidermal growth factor
- Transforming growth factor-alpha
- bFGF (basic fibroblast growth factor)
Following activation, angiogenesis may occur through one of the following processes:
Sprouting angiogenesis - Sprouting angiogenesis refers to the formation (or sprouting) of vessels from the wall of already existing vessels. Here, endothelial cells first migrate to the site (on the wall of the existing vessel) where they proliferate and organize in a manner that results in the formation of a new endothelial tube.
Here, growth factors like FGFs and VEGFs serve to activate and influence the proliferation of cells, in this case endothelial cells, at a given region of existing vessels.
In general, this type of angiogenesis occurs through the following stages:
· Activation - by signaling factors (e.g. VEGFs)
· Breakdown of the enveloping basement membrane of the endothelial cells
· Tip cell formation to guide sprouting of the new vessel (breakdown of the basement membrane is essential for this to occur)
This type of angiogenesis may occur in response to several factors including tissue demand for oxygen, and inflammation, or injury, etc.
At the site of vessel formation, enzymes are involved in the degradation of the basement membrane which allows new endothelial cells to come in contact with the vessel at the point where the new vessel is to be formed. Here, the proliferation of endothelial cells promote the development/elongation of the new vessels.
Non-sprouting angiogenesis (Intussusceptive angiogenesis) - The second type of angiogenesis is known as non-sprouting angiogenesis and is characterized by vessel formation through the emergence of a transluminal pillar.
Like sprouting angiogenesis, this type of angiogenesis also occurs through several important stages that include:
· Protrusion of endothelial cells into the lumen of a capillary
· Formation of interendothelial junctions by endothelial cells - protruding endothelial cells meet to form a strengthened interendothelial junction
· Reorganization of the junctions (interendothelial junction)
· Perforation - This is the result of the inter endothelial junctions reorganizing
· The pericytes, interstitial tissue, and myofibroblast invade the tissue bridge (between the two newly formed vessels)
Role of Endothelial Progenitor Cells in Cancer Angiogenesis
For a long time, it was thought that only the activation of mature endothelial cells resulted in the development of new blood vessels through angiogenesis. However, it's now understood that endothelial progenitor cells can be activated and recruited during angiogenesis.
For instance, following an injury (e.g. an injured retina), endothelial progenitor cells can be recruited from the bone marrow to the injured site. Proliferation and differentiation of these cells allow them to contribute to the vascularization process which in turn promotes the healing process.
By increasing vascularization to the site of injury, blood circulation of the site is improved which allows for such cells as platelets among other cells of the immune system to be transported to the site where they can promote healing.
While endothelial progenitor cells play an important role in healing through their role in angiogenesis, new findings have associated these cells with different types of cancers (breast cancer, lymphoma, etc).
Like all the other cells in the body, cells in a tumor also require nutrients and oxygen, etc to continue growing and dividing. This, then, means that new vessels have to be formed in order to continue supplying these cells.
To achieve this, tumors produce various growth factors that can activate angiogenesis. Two of the best examples of such factors are VEGF-A and bFGF. Following the release of these factors, endothelial progenitor cells are recruited from the bone marrow, while those residing in the tumor are activated, to the affected region.
Research studies have shown these cells to play several roles most important of which are producing new angiogenic growth factors and/or differentiate and give rise to mature endothelial cells.
While tumor cells are capable of producing factors that recruit endothelial progenitor cells, the environment in which these cells reside also contributes to this process. As mentioned, the region in which tumors start developing is not well vascularized.
For this reason, it's characterized by hypoxic conditions. This has been shown to influence the release of matrix metalloproteinases (MMPs) which in turn ruptures the extracellular matrix thus contributing to angiogenesis and spread of cancer cells. Following the rupture of the extracellular matrix, various compounds released contribute to the proliferation and differentiation of the endothelial progenitor cells thereby contributing to vascularization.
While the release of factors like VEGF-A plays an important role in recruiting endothelial progenitor cells; they have also been shown to prevent the recognition of tumor cells by immune cells. As a result, tumor/cancer cells can continue proliferating and spreading to other parts without being detected and destroyed.
In the tumor microenvironment, endothelial progenitor cells undergo differentiation to produce endothelial cells that are involved in angiogenesis (this may occur through the methods described above).
Here, vascularization, commonly referred to as neovascularization because it occurs postnatally, supplies the tumor with oxygen and nutrients required for continued growth and proliferation. As well, it also allows cancer cells to be spread to other parts of the body through the circulatory system.
Cardiovascular Disease
Cardiovascular disease is a general term used to refer to conditions that affect the heart or the blood vessels that transport blood to and from different parts of the body. Therefore, it can be used to refer to such conditions as angina, heart failure, and heart valve issues, etc.
As mentioned, the development of tumors has been associated with increased vascularization (angiogenesis). Cardiovascular disease and cardiovascular risk factors, on the other hand, have been shown to have the opposite effect on these cells.
As a factor that increases the risk of cardiovascular disease, aging has made it possible to understand the impact of cardiovascular disease and risks on endothelial progenitor cells. As compared to younger individuals, where the risk of cardiovascular disease is lower, studies have shown endothelial progenitor cells to have shortened telomeres among older individuals.
This has also been shown to be the case among patients with cardiovascular diseases which explains why patients generally have a lower number of endothelial progenitor cells. Even when these cells are activated, they are likely to undergo proliferative senescence and consequently more susceptible to apoptosis. This further reduces the number of endothelial cells over time.
For individuals with hypertension (a risk factor for cardiovascular disease), the number of endothelial progenitor cells in circulation has also been shown to be lower compared to that of healthy individuals.
Treatment of hypertension using drugs that inhibit the renin-angiotensin system allows the number of these progenitors to increase which is evidence that the condition is responsible for reduced functions and number of these cells.
Due to increased concentration of angiotensin II, a hormone that contributes to high blood pressure through the constriction of blood vessels, the function of telomerase in endothelial progenitor cells is reduced which increases the rate of senescence of the cells.
By affecting the functions and/or the number of endothelial progenitor cells, cardiovascular disease, and risks associated with the conditions directly or indirectly affects angiogenesis/neovascularization. This can increase the risk of other cardiovascular-related conditions like stroke and cardiac arrest given that the supply of oxygen to tissues and organs is reduced.
* While many studies have shown various cardiovascular disorders to affect the function, activities and the number of endothelial progenitor cells, it's worth noting that some of the disorders have been associated with an increase in these cells.
For instance, based on some findings, this has been shown to be the case with myocardial infarction
Engineered Tissue
As the name suggests, engineered tissue is a type of tissue that has been modified (using given cells and biochemical factors, etc) for the purposes of restoring, maintaining, and improving tissue functions or functions of an entire organ.
Because of the ability of endothelial progenitor cells to give rise to endothelial cells which are involved in neovascularization, they present a big potential for vascularizing engineered tissues.
Using these cells, it's possible to vascularize engineered tissue in different types of animals and allow them to not only survive, but also function as they would in healthy individuals. While this is one application of endothelial progenitor cells, researchers have also been using endothelial progenitor cells from donors to create vessels.
Using endothelial progenitors, researchers from Pohang University of Science and Technology among a few other Institutions were able to create tissue-engineered bio-blood vessels to treat ischemic disease. This involved using vascular-tissue-derived decellularized extracellular matrix from porcine aortic tissue among other essential components to ensure successful results.
This has proven beneficial not only for therapeutic purposes (treatment of given diseases), but also for the recovery and improvement of various tissues/organs in the body.
In some animals, the vessels have not only been successfully introduced, but also helped reinforce neovascularization in sites where the vessels were transplanted. Lastly, the other important area in which endothelial progenitor cells have been used (in tissue engineering) is for improving compatibility of vascular grafts.
A good example of this is in surgery that entails re-routing blood supply or replacement of blood vessels (e.g. vein replacement). In order to overcome the issue of tissue rejection, endothelial progenitors can be used to engineer new, compatible vessel tissue.
See also: Hematopoietic Progenitor Cells, Neural Progenitor Cells
Return to Progenitor Cells main page
Return to learning about Stem Cells
Return from Endothelial Progenitor Cells to MicroscopeMaster home
References
Erica B. Peters. (2018). Endothelial Progenitor Cells for the Vascularization of Engineered Tissues.
H. Chopra, M. K. Hung , D. L. Kwong, C. F. Zhang, and E. H. N. Pow. (2017). Insights into Endothelial Progenitor Cells: Origin, Classification, Potentials, and Prospects.
Naosuke Kamei, Kivanc Atesok, and Mitsuo Ochi. (2017). The Use of Endothelial Progenitor Cells for the Regeneration of Musculoskeletal and Neural Tissues.
Mervin C. Yoder. (2012). Human Endothelial Progenitor Cells
Suzanne M. Watt, Athanasios Athanassopoulos, Adrian L. Harris and Grigorios Tsaknakis. (2010). Human endothelial stem/progenitor cells, angiogenic factors and vascular repair.
Links
https://www.rndsystems.com/research-area/endothelial-progenitor-and-endothelial-cell-markers
Find out how to advertise on MicroscopeMaster!