What is Cellular Metabolism?
The 3 stages of Cellular Metabolism
Cellular metabolism refers to the chemical reactions that take place within cells. In eukaryotic cells, these reactions produce the energy required to maintain homeostasis among other important functions (e.g. metabolic turnover, cell division, contraction, etc). As such, cellular metabolism directly contributes to processes relating to growth, reproduction, and structural maintenance, etc.
Depending on the needs of the cells and functions, the rate of chemical reactions is either increased or decreased with numerous pathways synthesizing and breaking down cellular components.
Some of the enzymes involved in these reactions include:
- Hexokinase
- Pyruvate kinase
- Phosphofructokinase
- Phosphoglycerate mutase
- Phosphotriose isomerase
- Succinyl-CoA synthetase
- Citrate synthase
- Fumarase
Stages of Cellular Metabolism
While the breakdown of glucose to produce ATP (chemical energy) in cellular metabolism is continuous process, it's generally divided into three main stages that include:
1/ Glycolysis
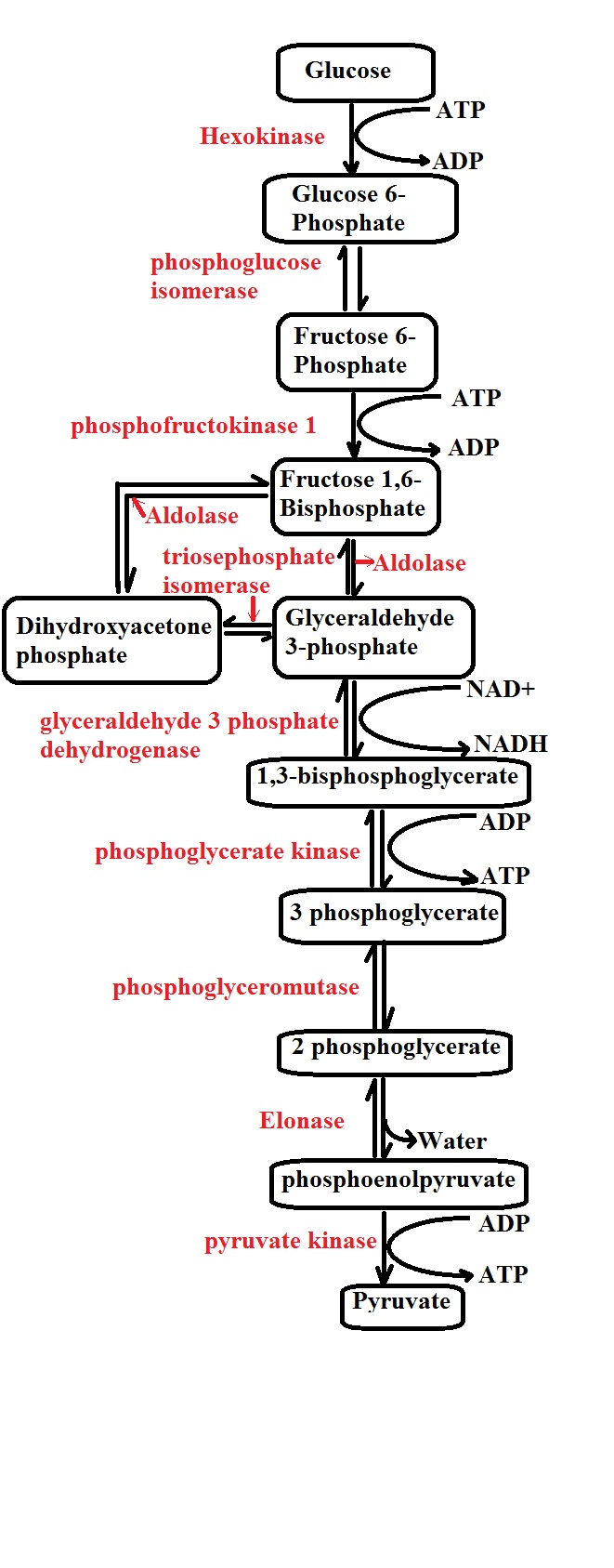
Generally, glycolysis may be described as the oxidation of a glucose (a hexose with six carbons) molecule to produce two molecules of pyruvate (each pyruvate molecule consists of three carbons).
This involves a number of important steps that will be described below in detail:
Given that glucose molecules cannot simply diffuse into the cell, they have to be transported by membrane proteins known as glucose transporters (GLUT) through a process known as facilitated diffusion.
While the majority of these transporters are not insulin dependent, those found in muscle and fat cells to be insulin-dependent.
Step 1: Phosphorylation 1- The first step of glycolysis is known as phosphorylation and takes place in the cell cytoplasm. As the name suggests, phosphorylation involves the addition of a phosphate to the glucose molecule which allows it to proceed to the next step.
Here, the phosphate added to the sugar molecule is derived from the breakdown of ATP in the cell which not only converts ATP to ADP and a phosphate, but also produces the energy required to add the phosphate to the sugar molecule.
This process is facilitated by the enzyme glucokinase in the liver and pancreas, while hexokinase is involved in the process in the muscles and converts the glucose into glucose-6-phosphate.
* Phosphorylation of glucose is important in that it creates a negative charge on the molecule which traps it in the cell thus preventing it from being transported out of the cell.
Step 2: Isomerization of glucose-6-phosphate to fructose-6-phosphate - The next step of glycolysis involves the conversion of the glucose-6-phosphate to fructose-6-phosphate. While they are both six (6) carbon sugars, the fructose phosphate is different from the glucose given that it's a ketone.
The carbon in the carbonyl group is bound to additional two carbons while glucose-6-phosphate is in the form of an aldehyde - the carbon atom in the carbonyl group is bound to a hydrogen and carbon atom.
The isomerization process is catalyzed by the enzyme phosphohexose isomerase (also known as phosphoglucose isomerase or Glucose-6-phosphate isomerase) and requires magnesium ion.
* While fructose-6-phosphate can be converted back to glucose-6-phosphate, the next step is irreversible. For this reason, the molecule is said to have committed to the glycolysis once it enters this next step.
Step 3: Phosphorylation 2 - In the third step, ATP is again required for the phosphorylation of fructose-6-phosphate to produce 1,6-bisphosphate (fructose diphosphate). As is the case with the first phosphorylation, ATP is again broken down to produce ADP and a phosphate.
Using the energy produced by the ATP, the enzyme phosphofructokinase type 1 adds a phosphate to fructose-6-phosphate which results in the production of 1,6-bisphosphate.
* Unlike fructose-6-phosphate, 1,6 -bisphosphate has two phosphates given that another phosphate is added during the third step (phosphorylation 2)
* The term "bisphosphate" refers to the fact that there are carbon atoms between the phosphates.
Step 4: Cleavage of fructose-1,6-bisphosphate - The fourth step involves the splitting of fructose-1,6-bisphosphate to produce dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (GA3P).
Here, the enzyme aldolase is involved in cleaving fructose-1,6-bisphosphate into the two three-carbon molecules. Although both the two molecules are three-carbon molecules, only one of the two (glyceraldehyde-3-phosphate) can proceed to the next step of glycolysis.
It's worth noting that either molecule can be transformed/converted to the other (isomerization). The enzyme responsible for this is known as triose-phosphate isomerase. In the event that the body has a high demand for energy, more dihydroxyacetone phosphate molecules are converted to glyceraldehyde-3-phosphate by the enzyme so that they can enter the next step.
Step 5: Dehydrogenation and phosphorylation - In this step of glycolysis, a molecule of glyceraldehyde-3-phosphate is converted to 1,3-bis-phosphoglycerate by the enzyme glyceraldehyde-3-phosphate dehydrogenase.
In the presence of the enzyme, NAD+ (nicotinamide adenine dinucleotide) reacts with glyceraldehyde-3-phosphate (2 molecules) which results in the molecule (glyceraldehyde-3-phosphate) losing hydrides. As a result, NAD+ is converted to NADH.
* Given that dihydroxyacetone phosphate is converted to glyceraldehyde-3-phosphate in the fourth step of glycolysis, then two molecules of NAD+ are converted to 2 molecules of NADH following the reaction of 2NAD+ with two molecules of glyceraldehyde-3-phosphate.
In addition to generating two molecules of NADH, the enzyme glyceraldehyde-3-phosphate dehydrogenase is also involved in the phosphorylation of the two molecules of glyceraldehyde-3-phosphate which results in the production of 1,3-bis-phosphoglycerate (each of the molecules has two phosphates).
Step 6: Dephosphorylation - During this step, 1,3-bis-phosphoglycerate undergoes dephosphorylation to form 3 phosphoglycerate in the presence of the enzyme by phosphoglycerate kinase.
In this step, two molecules of ADP react with the two molecules of 1,3-bis-phosphoglycerate in the presence of the enzyme to produce two molecules of ATP and two molecules of glyceraldehyde-3-phosphate.
Therefore, the enzyme phosphoglycerate kinase plays an important role in the liberation of phosphates from the two molecules of 1,3-bis-phosphoglycerate which results in the production/formation of two ATP molecules.
* In step 6, the enzyme phosphoglycerate kinase phosphorylates ADP to produce ATP. This is a very important step in glycolysis as it's the first time in this stage of cellular metabolism where energy in the form of two (2) ATP molecules is produced.
Step 7: In the next step, the enzyme phosphoglycerate mutase converts 3-phosphoglycerate to 2-phosphoglycerate which in turn converts to phosphoenolpyruvate (PEP) by the enzyme enolase.
Step 8: Dephosphorylation - In this step, phosphoenolpyruvate formed in the seventh step undergoes dephosphorylation which results in the production of two (2) additional ATP molecules.
Here, in the presence of the enzyme pyruvate kinase, two molecules of ADP react with two molecules of phosphate pyruvate phosphate results in the dephosphorylation of the two molecules of phosphate pyruvate phosphate. With the phosphorylation of the ADP molecules, two molecules of ATP and two molecules of pyruvate are produced.
At the end of step 8, pyruvate can enter one of two reactions depending on the presence or absence of oxygen. In anaerobic conditions (absence of oxygen), the pyruvate molecules are reduced in the presence of the enzyme Lactate dehydrogenase resulting in the production of lactic acid.
In this reaction, the two molecules of NADH are oxidized to produce two (2)molecules of NAD+. In the liver, the lactic acid can be converted to glucose or used to produce ATP molecules.
Due to the acidity of lactic acid, it can cause blood acidity by causing the pH to drop. In a case where a clinical test shows an increase in blood acidity, this can be indicative of such health conditions as myocardial infarction.
In the presence of oxygen (aerobic conditions), pyruvate enters the next stage of cellular metabolism.
2/ Krebs Cycle (Citric Acid Cycle or Tricarboxylic Acid Cycle)
In the presence of oxygen, pyruvate enters the Krebs cycle which is the second stage of cellular metabolism. However, before it actually enters this stage, it has to go through a transition stage also known as the preparatory phase.
Under aerobic conditions, pyruvate molecules are not converted to lactic acid and can therefore enter the mitochondria where they can go through an important transition step.
Decarboxylation - This transition step is known as decarboxylation and involves the conversion of the pyruvate molecules to Acetyl-CoA by the enzyme pyruvate dehydrogenase.
As the name suggests, this step involves the removal of carbon (the form of CO2) from the pyruvate by the enzyme pyruvate dehydrogenase.
The enzyme adds Coenzyme A to the 2 pyruvate molecules in the presence of NAD+ which not only results in the production of 2 Acetyl-CoA but also converts the NAD+ molecules to 2 molecules of NADH.
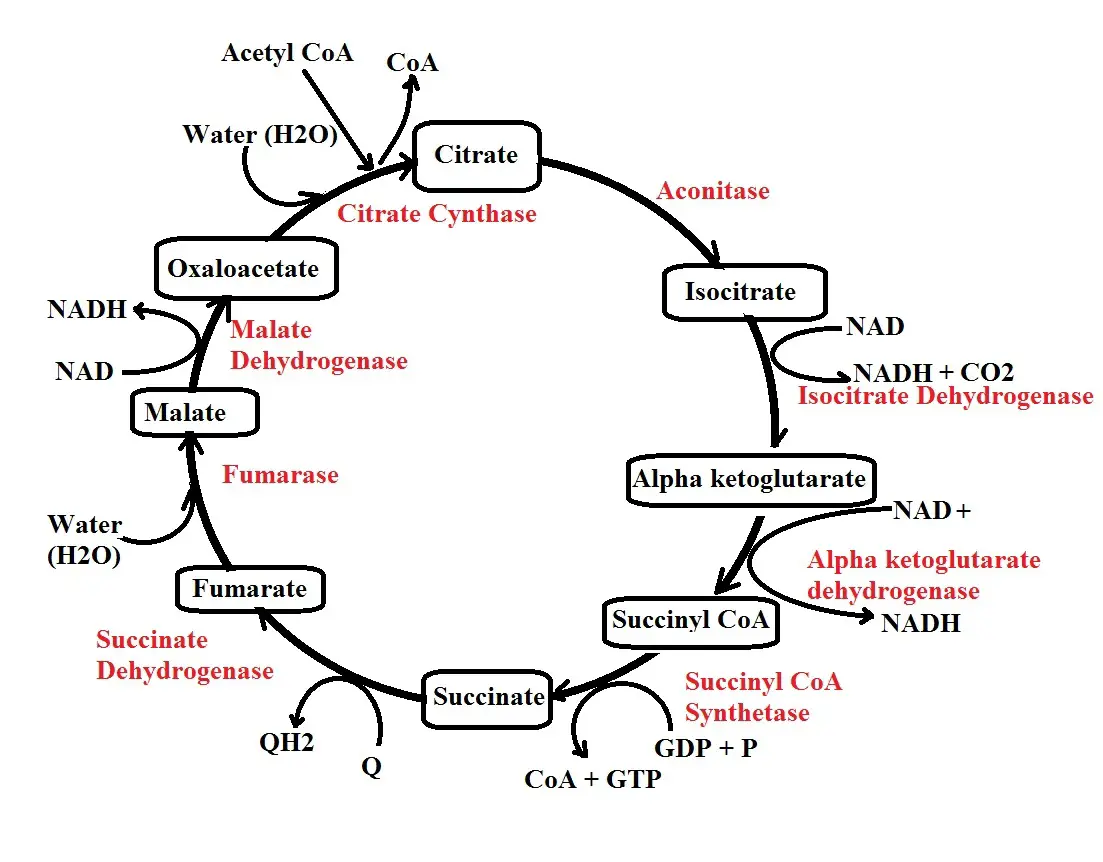
The following are the main steps involved in Kreb's cycle:
Step 1: Citrate synthesis - In the first step of Kreb's cycle, acetyl CoA produced during the transition stage combines with oxaloacetate (OAA) in the presence of the enzyme citrate synthase to form citrate.
As the name of the enzyme suggests, it's involved in the synthesis of citrate by combining acetyl CoA which is a two (2) carbon molecule and oxaloacetate, a four-carbon molecule.
* Step 1 of Kreb's cycle is highly regulated. Some of the molecules that regulate the function of the enzyme citrate synthase include ATP, NADH, and citrate. When there is a high amount of citrate (the molecule synthesized by the enzyme), it sends feedback limiting its activities.
Step 2: Isomerization - The second step is an isomerization reaction and results in the production of isocitrate. Here, the enzyme aconitase transforms the citrate to isocitrate by rearranging carbon molecules.
Here, it's worth noting that the process is reversible which means the isocitrate can be transformed back to citrate if need be.
* Isocitrate produced through the isomerization of citrate is less stable compared to citrate.
Step 3: Decarboxylation - In step 3, isocitrate is converted to alpha-ketoglutarate through a process known as decarboxylation. As the name suggests, this involved the removal of carbon from isocitrate in the form of carbon dioxide.
In the process, NAD+ is reduced to NADH and a hydrogen ion. This process is catalyzed by the enzyme isocitrate dehydrogenase. In this step, then, NAD+ reacts with isocitrate in the presence of the enzyme which reduces the NAD+ while converting the isocitrate to alpha-ketoglutarate.
In the presence of too much ATP, the enzyme involved in this reaction is limited thus reducing the production of alpha-ketoglutarate. However, high amounts of ADP promote the action of the enzyme thus enhancing its activities.
Step 4: Decarboxylation 2 - In step 4, alpha-ketoglutarate is converted to succinyl CoA by the enzyme alpha-ketoglutarate dehydrogenase. In this reaction, NAD+ reacts with alpha-ketoglutarate in the presence of the enzyme alpha-ketoglutarate dehydrogenase which again results in its reduction.
A carbon is also lost in the form of carbon dioxide resulting in the production of succinyl CoA. In a case where too much energy is produced in the cell, the molecule, succinyl CoA, binds to the enzyme thus limiting its activities. As a result, the production of succinyl CoA is reduced. Some of the other substances that inhibit the enzyme include NADH and calcium.
Step 5: Hydrolysis - In step 4, a CoA is added resulting in the production of succinyl CoA. In step 5, on the other hand, the CoA is removed resulting in the production of succinate.
The enzyme involved in this step is known as succinyl CoA synthetase and functions by stimulating the conversion of succinyl CoA to succinate. In this process, CoA is released along with a phosphate.
Here, a GDP (Guanosine diphosphate) molecule takes up the phosphate to form GTP (Guanosine triphosphate). However, GTP loses the phosphate to ADP which results in the production of ATP. This is known as substrate-level phosphorylation.
* In step 5, hydrolysis of GTP produced ATP.
Step 6: Fumarate - In step six (6) of the cycle, succinate is converted to fumarate by the enzyme succinate dehydrogenase. Here, FAD (flavin adenine dinucleotide) reacts with succinate in the presence of the enzyme which results in its reduction to FADH.
Step 7: Hydrolysis - In step 7, the enzyme fumarase is involved in the hydrolysis of fumarate to form malate.
Step 8: Production of Oxaloacetate - In this step, the enzyme malate dehydrogenase is involved in the conversion of malate to oxaloacetate. Here, NAD+ reacts with malate in the presence of the enzyme causing it to be reduced to NADH and a hydrogen ion. Once it's produced, Oxaloacetate can then enter the cycle by accepting another molecule of acetyl CoA as the cycle continues.
* With two molecules of Acetyl CoA, Kreb's cycle produces 4 carbon dioxide molecules, 6 molecules of NADH, 2 molecules of FADH2, as well as 2 ATP molecules. NADH and FADH2 molecules are important for the third and last stage of cellular metabolism.
3/ Electron Transport System (Electron Transport Chain)
The electron transport system/chain is the third and last stage of cellular metabolism and takes place in the folded, inner membrane of the mitochondria (cristae). This is a particularly important stage given that most of the ATP molecules are produced here.
This stage also includes several important steps of electron transfer that include:
Step 1: In the first step of the electron transport system, the NADH molecules come in contact with enzyme complex 1 which takes electrons from these molecules thus converting them from NADH to NAD+ (6 molecules).
Unlike NADH, FADH has a higher affinity for enzyme complex 2 and thus reacts with the enzyme releasing two electrons (2 FADH molecules release electrons to complex 2). This converts the 2 molecules of FADH to 2 molecules of FAD and protons.
Step 2: In step 2 of cellular metabolism, complex 1 releases the electrons it gained from NADH so that it can move from a high energy to a low energy level. This also causes a pore on the complex to open allowing for protons to be pumped out.
While complex 2 also releases electrons in order to switch to a lower energy state, it does not have pores and therefore protons cannot be pumped out. In step 2, all the electrons released by complex 1 and 2 are taken up by Coenzyme Q (also known as ubiquinone).
Step 3: One of the defining characteristics of Coenzyme Q is the fact that it is mobile and can therefore move within the cristae. This is important in this step as it allows the molecule to move in order to transfer the electrons. In this step, the molecule moves and transfers its electrons to enzyme complex 3.
Step 4: As with the other enzyme complexes in this system, enzyme complex 3 also changes to a higher energy level having received electrons. In this state, it has to release these electrons in order to switch back to a lower energy level.
Again, then, the electrons are transferred to another mobile molecule known as Cytochrome C. Like enzyme complex 1, this complex also has a pore than opens following the release of electrons thus allowing for protons to be pumped out into the intermembrane space.
Step 5: In step 5, the process is again repeated with Cytochrome C releasing the electrons in order to switch to a lower energy level. These electrons are then accepted by enzyme complex 4.
Step 6: In step 6, enzyme complex 4 releases the electrons it received thus moving from a high energy to a lower energy level. Here, however, the electrons released combine with oxygen and protons to produce water. The release of electrons here also causes a pore to open thus allowing protons to be pumped out.
* Up to this step, it's evident that the complexes (as well as other involved molecules) receive and release electrons thus creating a chain of electron transfer.
Here, the complexes switch to a higher energy state each time they receive the electrons and have to release these electrons in order to switch back to a more stable, lower energy level.
As a result of releasing protons into the intermembrane space, there is a high accumulation/concentration of these electrons in this space compared to the mitochondria complex.
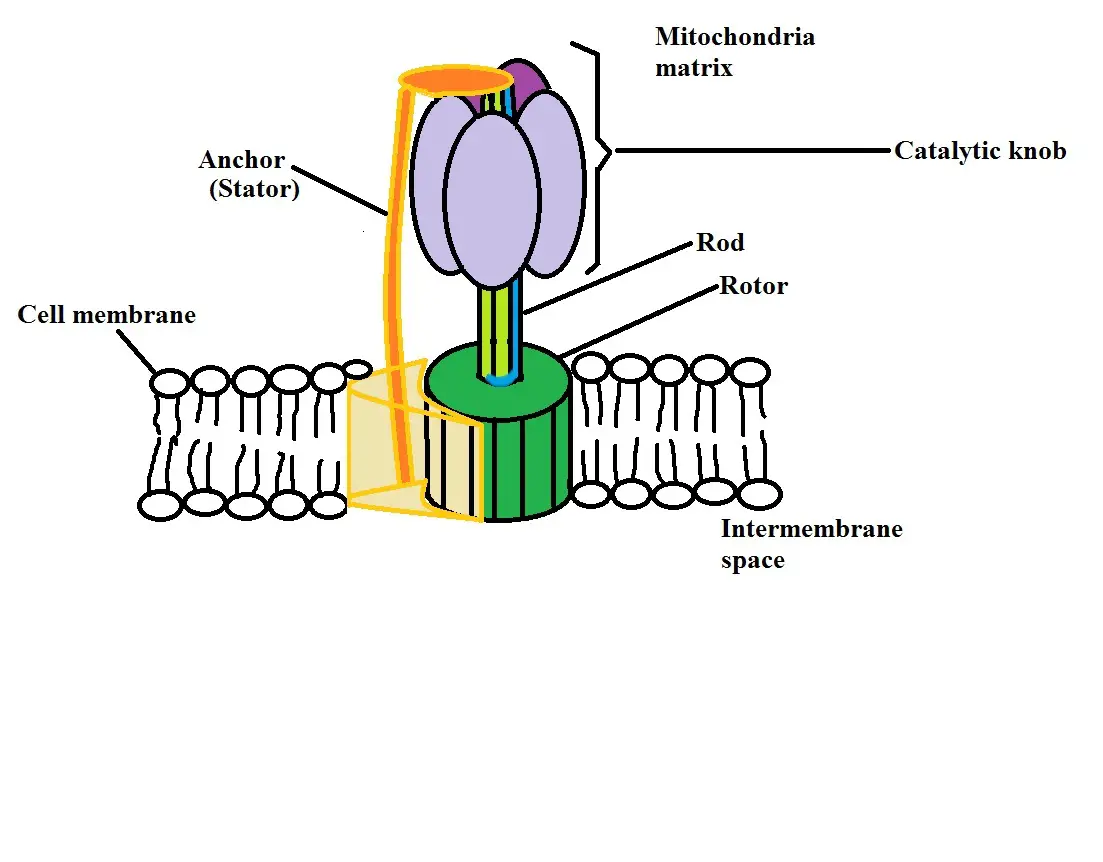
Because of this difference, a gradient is created which means that they are promoted to move from the area of high proton concentration (in the intermembrane space) to the area of lower proton concentration (the mitochondria matrix). To do this, the protons move through a special structure known as ATP synthase.
Here, protons pass through the stator part of the structure which allows them to move towards the mitochondria matrix. As they pass through, this causes another part of the structure known as the rotor to start spinning. As it spins with the movement of protons into the matrix, the rotor starts to absorb potential energy.
This energy is then used by another part of the ATP synthase structure (the catalytic knob) to create ATP molecules from phosphates and ADP. The ADP and phosphates are contained in the catalytic knob and are used to produce ATP when potential energy is absorbed in the rotor.
Here, the process used to produce ATP from potential energy created through the movement of protons is known as Oxidative Phosphorylation.
* Under aerobic conditions, the three stages of cellular metabolism produce a total of 36 ATP molecules. Anaerobic conditions result in the production of 2 ATP molecules from glycolysis in particular.
Here, learn more about Cell Division, Cell Differentiation, Cell Proliferation and Pentose Phosphate Pathway
See articles on Cell Culture, Cell Staining and Gram Stain.
What are the Differences between a Plant Cell and an Animal Cell?
Check out information on Cell Theory.
Why is Cell Adhesion important?
Return from What is Cellular Metabolism? to MicroscopeMaster home
References
Christian M. Metallo and Matthew G. Vander Heiden. (2014). Understanding metabolic regulation and its influence on cell physiology.
Donald C Rizzo. (2000). Fundamentals of Anatomy and Physiology.
Jones and Bartlett Publishers. Chapter 7: Metabolism.
Michael Palmer. (2019). Human Metabolism. Lecture Notes.
Links
https://www.biologyonline.com/dictionary/catabolism
https://www.nature.com/scitable/topicpage/cell-metabolism-14026182/
Find out how to advertise on MicroscopeMaster!