Cryo-Electron Tomography
Resolution, Advantages and Advances
Overview
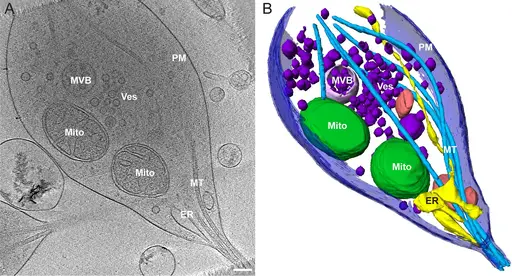
Also known as electron cryotomography, cryo-electron tomography is a form of cryo-electron microscopy used to produce three dimensional (3D) images of macromolecules at their near-native/physiological state. This is achieved by combining three core technologies namely, tomography, electron microscopy, and cryogenics.
Resolution
Currently, cryo-Electron Tomography is one of the highest resolving (2-5nm) techniques used for imaging macromolecules and cellular components in 3D at their physiological state. As such, the technique provides significantly higher resolution, particularly when compared to light microscopy which provides a resolution of about 400nm.
While this technology provides high resolution, a number of factors have been shown to have a direct impact on the resolution.
These include:
Sample thickness - Ideally, the specimen under investigation should be thin enough to allow the incident electron beam to pass through. Here, a thickness of about 0.5 microns is recommended.
During sample preparation, cells are first grown on electron microscopy grids. Gold EM grid is preferred because it's not toxic to cells. These cells are then rapidly frozen in order to preserve them in their natural state. This is achieved through the use of a vitrobot which performs automated vitrification.
One of the biggest advantages of vitrification (using liquid ethane at -180 degrees C) is that cells are frozen so fast that water molecules present do not have time to crystallize. In the process, the cells are preserved in their native/physiological state.
* If the sample is not cooled fast enough, crystallization can occur thus denaturing the sample.
To cut the specimen to the desired thickness, one of the methods used is known as FIB-million, focused ion beam milling. For this method, the surface of the region to be imaged is first coated with a film of platinum. This prevents this region from ion beam erosion.
In such devices as the Aquilos Cryo-FIB, the specimen is kept vitreous at cryo temperature. Once the specimen/cells are coated, an ion (e.g. Gallium ion beam) beam is used to remove materials from vitrified cells. As well, an electron beam serves to image the specimen.
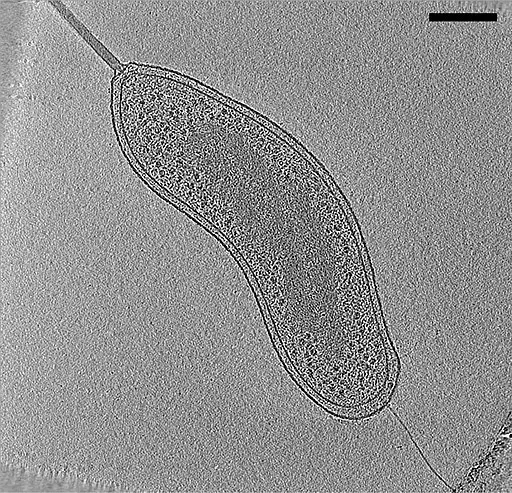
Here, the area to be removed is first marked allowing for the region of interest to be retained following excision by the ion beam. The sample of interest is then transferred to a second electron microscopy grid where it's fixed and milled again with an ion beam (at lower voltage) to produce a thinner section. The second phase of thinning produces a specimen that is thin enough to allow for an electron beam to pass through.
* The thin section is commonly referred to as lamella.
* Apart from focused ion beam, a cryo microtone is also used to cut the specimen and produce thin slices.
Electron Radiation Dose (electron beam dose)
The other factor that has a direct impact on resolution is the electron radiation dose. In electron microscopy, electron beams used can cause damage to the specimen. For this reason, given doses of the beam has to be used to avoid this. Generally, a low electron dose is recommended.
During imaging, the region of interest is first located using low magnification using a low electron dose. At the same time, the beam is focused on the areas adjacent to the region of interest before data collection starts. In doing so, technicians can significantly minimize the dose level that the region of interest is exposed to.
Sensitivity of the Detector
Poor detector sensitivity negatively impacts resolution. Recently, however, new methods have been used to enhance sensitivity. A good detector is characterized by high quantum efficiency and low read noise. However, all detectors add noise, which can be viewed as one of the main limitations of this type of microscopy.
Quality of Electromagnetic Lenses
In electron microscopy, electromagnetic lenses are used in place of glass lenses. These lenses consist of a coil of copper wire within pieces of iron poles. By passing a current through the coils, a magnetic field is created which in turn fills the center of the iron poles with a strong magnetic force.
It's this force that serves to focus the electron beam in a given direction (to focus the beam onto the specimen). Given that the lenses used in this technique play an important role in focusing the beam, the quality of these lenses has a direct impact on resolution.
In a scenario where the factors mentioned above are ideal, high resolution in cryo-electron tomography is made possible by the use of an electron beam (rather than photons used in light microscopy).
Here, the electron beam is passed through a vacuum before reaching the specimen. This is particularly important in this technique given that air would result in electron scattering.
It's also worth noting that this method uses a form of electron microscopy known as transmission electron microscopy (TEM). This technique is characterized by electrons traveling at a very high speed in order to pass through the specimen.
Resolution is also dependent on the size of the wavelength so using electron beams also makes it possible to get higher resolution because the wavelength of electrons is much shorter compared to that of the visible light.
* Whereas the wavelength of the visible light spectrum ranges from 380 to 700 nanometers, the wavelength of electrons used in this technique ranges from 0.00335 to 0.00197 nanometers.
Some of the other benefits of electrons used in electron microscopy include:
- Lighter and thus easier to focus
- The electromagnetic lens used makes it possible to easily focus the electrons
- Generate large amounts of information
* An optical microscope uses optical light (photons), it's not possible to focus light to a point that is smaller than the wavelength of light. For this reason, points separated by a given distance cannot be resolved.
Given that electrons have a much shorter wavelength, it becomes possible to focus the beam at a given point and resolve between a shorter distance between two points. Therefore, the wavelength of electrons makes it possible to obtain higher resolution using a transmission electron microscope in cryo-electron tomography.
Subtomogram Averaging
Subtomogram averaging can be described as the analysis and alignment of a set of tomograms in order to generate an average tomogram. During imaging, multiple images of a given structure present in a set of tomograms used to produce a clear or more enhanced 3D structure that is near-atomic resolution. Therefore, subtomogram averaging is an excellent approach to getting a more enhanced image of a structure.
Generally, the process starts with the identification and location of the particles of interest (these may include protein complexes etc). Once the particles have been located in the tomograms, the next step involves defining their coordinates.
This is commonly known as particle picking and can be performed through a number of approaches that include:
Manual picking - Manual picking of the particles is a tedious strategy and therefore not commonly used. Here, researchers have to manually pick the particles of interest in 3D volumes which can be time-consuming especially for sub-nanometer structures.
Particle picking relative to a given support geometry - In this approach, coordinates for picking particles are first defined. Subvolumes can then be extracted and averaged so as to obtain a starting reference for aligning sub-volumes. This has proved useful in defining particles along with various structures (e.g. microtubules).
Automated particle picking - Unlike the other strategies, this approach involves the use of a template for particle picking. Here, the template is first rotated (using a computer) and then cross-correlated against positions in the tomogram. The positions and angles that correspond to the highest cross-correlation values are then ranked while subtomograms are extracted from highly ranking locations.
* More recently, a good number of researchers have been using supervised particle picking through pre-trained neural networks. In this strategy an annotated training dataset consisting of regions of the tomograms (with regions of interest) is first provided. The neural network is then trained using these inputs to pick particles.
Following particle picking, subtomogram may be performed through the following steps:
Cross correction maximization - In this step, a common signal is first located through an iterative process. Each of the subtomogram is then aligned against a reference volume known as the template.
By determining the translation and rotation that is needed for the subtomograms, it becomes possible to maximize similarity of both the template and subtomograms. In the averaging stage, these parameters are used to produce an enhanced template.
Similarity measurement - In this stage, the constrained cross-correlation function is used to measure similarities of the template and particle. A mission wedge is then used on the template thereby filtering it to frequencies that are present in the subtomogram. Here, a real space mask is also often used for the purposes of limiting similarity measurement to the region of interest.
Subtomogram alignment - This third step involves the alignment of the particles. Most of the computational time is allocated towards searching the angles and shifts that maximize similarities between the particle and template.
Averaging step - In this step, the particles are averaged so as to create a volume to be used as reference for the next iteration and so forth. By the end of this step, there is evident increase in resolution as the resulting averages are ultimately placed at corresponding coordinates in the full tomogram.
Advantages
Cryo-electron tomography has a number of advantages that make it one of the most useful tools in various fields like molecular biology.
Some of the main advantages of this technique include:
High Resolution
As mentioned, cryo-electron tomography involves the use of transmission electron microscopy (as compared to scanning electron microscopy, transmission electron microscopy is used to pass electrons through the specimen).
The use of electrons, in place of photons, allows this technique to produce a high resolution. This is particularly beneficial given that it makes it possible to get a better view of the sample.
As compared to a light microscope, it's possible to get a better view of the structure of various cellular components. This has been of significant benefit to students of molecular biology as it has provides a better understanding of molecular structures etc.
3D Imaging
One of the other advantages of cryo-electron tomography is that it produces a 3D image of the structures under investigation. This is achieved by recording a series of images of the sample (targeted region) as the grid (which holds the sample) is tilted.
By tilting the grid, the specimen, lamella, is also tilted incrementally allowing for images to be recorded from different angles. These images are then combined on a computer to produce a 3D model of the structure under investigation.
Through advances in cryo-electron tomography, researchers have been able to improve various parts of the device to ensure excellent results. For instance, given that the technique involves imaging of a very small region of interest, the sample may move out of the field of view as imaging continues.
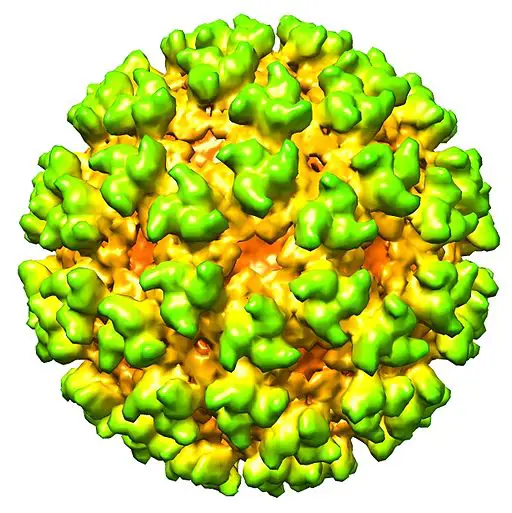
For this reason, a tracking region of the grid was introduced to ensure that the right/intended region of the specimen is imaged. As a result, the final image of the specimen produced is the right image. This has made it possible to determine the general structure of various macromolecules and organisms (e.g. structure of viral capsid).
Physiological State
One of the other advantages of cryo-electron tomography is that the technique has made it possible to study various organisms and macromolecules at their near-native/physiological state. In the past, such techniques are X-ray crystallography were used to determine the structural biology of these organisms (e.g. viral particles) and macromolecules. However, because of various limitations, they now prove inferior when compared to cryo-electron tomography.
While X-ray crystallography made it possible to study biological structures, the technique involved crystallization of the sample which caused damage to the specimen. These methods made it possible to study structures in their physiological state. However, in the case of cryo-electron tomography, these limitations are overcome making it possible to study biological structures at their physiological state (or at least near their physiological state).
For example, while freezing is necessary, in this technique, technicians are careful to ensure that water molecules on these surfaces (region of interest) are not crystallized during sample preparation. Here, care is taken to preserve the specimen throughout the process so that the structure is not compromised.
During imaging, care is also taken not to expose the structures to heavy doses of the electron beam in order to maintain the integrity of these structures. In general, this technique has proved more effective when it comes to studying macromolecules and cells, etc at their physiological state.
* While care has been taken to preserve the integrity of biological samples, electrons can still cause inelasticity or break various structural bonds of the samples. And so, the technique is not yet perfect for studying biological components.
Advances in Cryo-Electron Tomography
While cryo-electron tomography is still a relatively new microscopy technique, it continues to experience significant improvements with advancements that have resulted in improved detector sensitivity, automated data acquisition, enhanced image reconstruction, and the implementation of phase plates.
In particular, many researchers are focusing on phase plates and spherical aberration correctors in order to improve image contrast. This has seen gradual improvements making it useful in various biology sub-disciplines.
In microbiology, it has not only allowed researchers to study cellular characteristics of various organisms, but also the molecular structure of cellular components and various macromolecules. It has gained significant attention across the world and continues to replace some of the techniques that were previously used.
Check out a great page on Nanotechnology here
Scanning (SEM) - Learn about the SEMs high-resolution, three-dimensional images which provide topographical, morphological and compositional information making them invaluable in a variety of science and industry applications.
Transmission (TEM) - check out one of the most powerful microscopic tools available to-date, capable of producing high-resolution, detailed images 1 nanometer in size.
Cryo-Electron - is a type of transmission electron microscopy that allows for the specimen of interest to be viewed at cryogenic temperatures. Check it out.
Virtual - provides a simulated microscope experience via a computer program or Internet website for both educational and industrial applications and are easily operated and accessible.
Take a look at how Electron Microscopy compares to Super-Resolution Microscopy.
Taking a look at Viruses under the Microscope and answering the question, what are viruses?
As well as Atom under the Microscope and DNA under the Microscope
Electron microscopy (SEM and TEM) images of SARS-CoV-2 - Covid 19
Return to learning about other Microscopy Techniques
Return from Cryo-Electron Tomography to MicroscopeMaster home
References
Gavin E. Murphy & Grant J. Jensen. (2018). Electron Cryotomography.
Marina Serna. (2019). Hands on Methods for High Resolution Cryo-Electron Microscopy Structures of Heterogeneous Macromolecular Complexes.
Kendra E. Leigh. (2019). Subtomogram averaging from cryo-electron tomograms.
Peter Ercius, Osama Alaidi, Matthew J. Rames, and Gang Ren. (2015). Electron Tomography: A Three-Dimensional Analytic Tool for Hard and Soft Materials Research.
Roman I. Koninga, Abraham J.Kostera and Thomas H. Sharp. (2018). Advances in cryo-electron tomography for biology and medicine.
Rebecca F. Thompson et al. (2016). An introduction to sample preparation and imaging by cryo-electron microscopy for structural biology.
Links
Find out how to advertise on MicroscopeMaster!