What are Glycoproteins?
Importance
Where are they found?
Definition
Glycoprotein refers to proteins in which oligosaccharide chains (glycans) are covalently attached/bound to the amino acid side chains (polypeptide backbones). In different types of organisms, these molecules are formed through a process known as glycosylation.
Here, the number and type of sugar molecules added to the glycosylation site on the protein varies depending on the type and function of a glycoprotein. This is made possible by the fact that proteins have multiple sites for glycosylation allowing for various glycosidic linkages.
Examples of Glycoproteins
- Mucins
- Ceruloplasmin
- Collagens
- Calnexin
- Calreticulin
- Selectin
What are Glycoproteins?
As mentioned, glycoproteins are protein molecules (polymers) with covalently linked carbohydrates. Here, glycosylation has been shown to occur following the production/synthesis of proteins (post-transitional) which means that the carbohydrate component of glycoproteins is added following protein synthesis. However, carbohydrates can be added as the protein molecule continues growing in some cases.
For this reason, the carbohydrate is considered to contribute to the main biological functions of the molecule. Carbohydrates found in glycoproteins are referred to as oligosaccharides; polymers that consist of between 3 and 10 monosaccharides.
Generally, oligosaccharides are not found freely in cells. Rather, they exist as complex molecules linked to proteins (N-linked or O-linked). In human beings, there are several types of sugars (monosaccharides) found in oligosaccharides.
These include:
- Hexose sugars: Galactose, mannose, and glucose
- Aminohexose: N-Acetylgalactosamine, N-Acetylglucosamine
- Deoxyhexose: Fucose
- Pentose: Xylose
- Sialic acid: N-Acetylneuraminic acid
Glycosylation
Glycosylation refers to the process through which carbohydrate moieties are added to a protein molecule to form a glycoprotein. In eukaryotic cells, this process starts in the endoplasmic reticulum (ER) and continues in the Golgi apparatus.
While this generally involves the linkage of carbohydrates to proteins, glycoproteins are divided into two main categories based on how this linkage occurs.
* Oligosaccharides are linked to proteins through specific amino acids. These include asparagine amino acid, serine amino acid, as well as threonine amino acid.
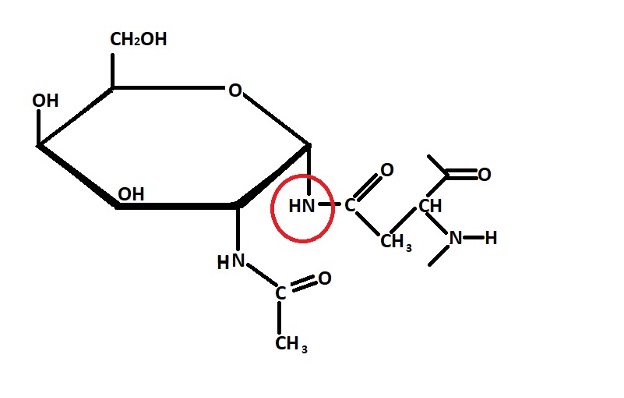
N-linked glycoprotein - N-linked glycoproteins are formed through N-linked glycosylation. Here, carbohydrates are linked to the nitrogen on the side chain of the amino acid asparagine.
In N-linked glycosylation, the carbohydrate (oligosaccharide) to be linked to the asparagine residue of a protein is assembled in the endoplasmic reticulum. The assembly process starts with the addition of 2 N-acetylglucosamine and 5 mannose residues to dolichol phosphate in the presence of several cytoplasmic enzymes. These enzymes mediate the transfer of monosaccharides from sugar nucleotides.
The molecule is then transferred from the membrane of the endoplasmic reticulum to the lumen where sugar molecules are again added. Ultimately, the process results in the production of a large oligosaccharide molecule that is attached to dolichol phosphate.
Once the carbohydrate is fully assembled, it's transferred to the site of a growing polypeptide chain so that it can be linked to the asparagine residue of the protein. Generally, this involves the recognition of a specific sequence of activated sugars.
Once the carbohydrate molecule is linked to the asparagine residue of the protein, dolichol pyrophosphate is recycled to dolichol phosphate in the presence of the enzyme phosphatase so that the process can be repeated with a newly assembled oligosaccharide molecule.
Structure of an N-linked Glycoprotein
* While N-linked glycosylation is common in virtually all eukaryotic organisms, it has been shown to be absent in prokaryotes.
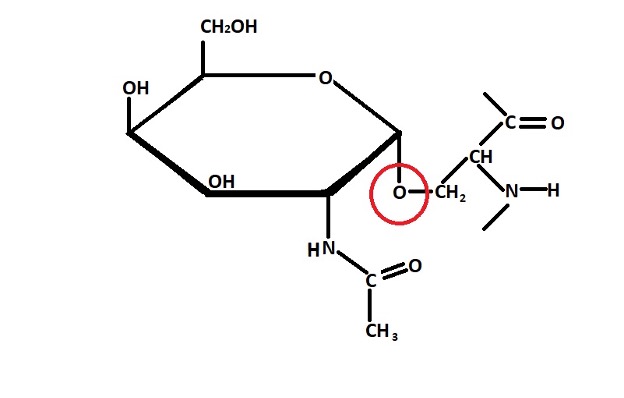
O-linked glycoproteins - Unlike N-linked glycoproteins, O-linked glycoproteins are formed through a process known as O-linked glycosylation. The carbohydrate is linked to the oxygen atom on the side chain (located in the hydroxyl group) of serine of threonine amino acid.
As is the case with N-linked glycosylation, the oligosaccharide molecule to be attached to the protein is first assembled and transferred from nucleotide precursors. Once the oligosaccharide has been assembled, it's then transferred and linked to the hydroxyl group of threonine or serine of a protein in the Golgi complex.
This process, however, may involve several unique steps compared to those that are involved in N-linked glycosylation.
In Glycophorin, for instance, the enzyme GalNAc transferase first mediates the transfer of N-acetylgalactosamine to the hydroxyl group of the protein amino acid residue (serine or threonine) from UDP – N-acetylgalactosamine.
This molecule is then transferred from the rough endoplasmic reticulum (or the cis-Golgi network) to the trans-Golgi vesicles where sugars (galactose residue) are added to the N-acetylgalactosamine linked to the protein in the presence of the enzyme trans-Golgi galactosyltransferase.
Structure of an O-linked Glycoprotein
In general, the glycosylation process may be summarized as follows:
· In the cytoplasmic side of the endoplasmic reticulum, proteins are first produced/synthesized by ribosomes (ribosomes located on the surface of rough endoplasmic reticulum).
Once they are produced, these proteins move into the lumen of the endoplasmic reticulum where a given set of enzymes link oligosaccharides to the proteins through N-linkages - Here, it's worth noting that N-linkage of oligosaccharides takes place within the lumen of the endoplasmic reticulum.
· The proteins are then transported to the Golgi complex from the lumen of the endoplasmic reticulum where the N-linked sugars are further modified. For some of the proteins, this is the site of O-linked glycosylation.
· When the two types of glycoproteins are fully modified within the Golgi complex, they are released and transported to the cell membrane where they are involved in various functions.
Significance of Glycosylation
As the process through which oligosaccharides are added to a protein, glycosylation is important for several reasons.
These include:
- Helps protect and stabilize some proteins
- Allows some proteins in the body to effectively perform given functions
- Important for blood grouping
- Results in the production of different types of glycoproteins
Where are Glycoproteins found?
Cell membrane
In various organisms, both eukaryotic and prokaryotic, as well as viral particles, glycoproteins can be found on the cell membrane (cell envelope) and thus in various tissues.
Here, they are part of the cell envelope components and are involved in various functions. For the most part, these molecules extend outwards (extending into the extracellular matrix in multicellular organisms) which allows them to effectively carry out their functions.
These functions are discussed below in detail.
Plasma
Some glycoproteins can be found in plasma. Good examples of these are mucopolysaccharides, albumin, embryo globulin, and mucoproteins among others. Generally, these glycoproteins are characterized by galactose and mannose in the oligosaccharide side chain.
Different types of plasma glycoproteins serve various functions. Mucopolysaccharides, for instance, have been shown to be an important protein that has not only been associated with the pathogenesis of given disease agents, but also allows for elevation of different types of diseases.
* Some glycoproteins are secreted within the cell and released into the extracellular matrix.
Importance
Protect the epithelial cells
Secreted by epithelial cells, mucins are heavily glycosylated proteins that contain many oligosaccharides because they have a high number of threonine and serine residues.
In various multicellular organisms, mucins are the primary components of the mucous membrane and can therefore be found lining various parts of the body including the nasal cavity, bronchioles, the gastric mucosa as well as the uterus, etc.
For the most part, mucins are produced and released into the extracellular matrix. However, some are retained in the cellular membrane.
Due to the presence of modified sugars on the glycoproteins, studies have shown the molecule to have a negative charge, due to the negative charge of the modified sugar molecules, that attracts water molecules.
For this reason, mucin is able to lubricate the epithelial tissue. This is also important given that the carbohydrates (of mucin) become sufficiently sticky to trap various pathogenic agents. This prevents these organisms from coming in contact or entering the cell thus preventing an infection.
* By forming a physical barrier on the surface of epithelial tissue, mucin has also been shown to help protect cells of these tissues from stress-induced damage.
They act as hormones
Glycoproteins are collectively known as gonadotropins.
They have been shown to be some of the most complex hormones and include:
Follicle-stimulating hormone (also known as follitropin) - Produced in the pituitary gland, the follicle-stimulating hormone (FSH) is a gonadotropin that controls the functions of ovaries in females and testes in males. As such, it plays an important role in development during puberty.
While it regulates the functions of ovaries and testes, the hormone is itself under the influence of various hormones that are released from these organs. For instance, in men, the level of testosterone and inhibin (released from the testes) influence the production of FSH.
* While FSH influence the growth of eggs (ova) and contro/regulate the menstrual cycle in women, it activates the growth of testes and promotes the production of proteins from the Sertoli cells involved in the maturation of sperm cells in men.
* Glycoprotein hormones belong to the group of glycoproteins that are secreted and released from the cells of the anterior pituitary.
Luteinizing hormone (LH) - Like the follicle-stimulating hormone, luteinizing hormone (also known as lutropin) is synthesized and released from the cells of the pituitary gland (anterior part of the gland). Moreover, it also plays an important role in regulating the functions of ovaries (in women) and testes (in men).
While luteinizing hormone has some similarities to follicle-stimulating hormone, they have several differences. In men, for instance, the luteinizing hormone plays an important role in influencing the production of testosterone in the testes.
This is an important hormone that promotes sperm production. Moreover, it's also involved in the development of various male characteristics (e.g. deep vice and increased production of body hair, etc.).
In women, on the other hand, the luteinizing hormone is involved in the production of estradiol (sex hormone) from the follicles in the ovary as well as the release of mature eggs during ovulation.
Thyroid-stimulating hormone - Produced by thyrotrophic cells in the anterior pituitary gland, thyroid-stimulating hormone is also a glycoprotein. From the pituitary gland, the hormone is released into the bloodstream from where it regulates the production of various thyroid hormones (these include triiodothyronine and thyroxine). In doing so, thyroid-stimulating hormone indirectly influences various body activities including the metabolic rate and digestive functions among others.
Human chorionic gonadotropin - Like the other gonadotropins, the human chorionic gonadotropin hormone (hCG) is produced in the pituitary gland. In women, the hormone is especially common during pregnancy and can be found in both the blood and urine.
Given that it becomes detectable about 3 weeks following implantation of the embryo in the uterus, it helps inform mothers of the pregnancy. During pregnancy, the hormone promotes the production of estrogen and progesterone which maintain the pregnancy before birth.
* Apart from the hormones produced in the pituitary gland, there is a number of other glycoprotein proteins/hormones involved in other functions.
These include:
Erythropoietin (EPO) - Produced in the kidney, erythropoietin is a glycoprotein hormone involved in the production of red blood cells
Prothrombin - Also made in the liver, prothrombin is a glycoprotein involved in clotting. As such, it helps prevent excess bleeding
Cell recognition and adhesion
Cell to cell recognition refers to the ability of a cell to identify a neighboring cell. Here, glycoproteins play an important role in cell to cell recognition given that the glycoprotein of one cell can identify and respond to the oligosaccharide patterns (of glycoproteins) of another cell. It's also through this process that binding (adhesion occurs).
Here, cell recognition, following recognition of oligosaccharide patterns, allows glycoproteins to bind together in a manner similar to the lock and key hypothesis. This is also important for cell communication given that cells are able to respond following the identification and binding process.
Cell markers
The term cell marker refers to specific markers (molecules) expressed in different types of cells that allow the cell to be identified. Here, the cell can be identified based on the type of markers expressed. In the body, some glycoproteins act as markers and thus allow given cells to be identified based on these specific molecules.
Some of the best examples of glycoprotein markers are Glycophorins A and B. Generally, these are classified as sialoglycoprotein which means that the glycoprotein also consists of sialic acid. Found in the membrane of human erythrocytes, the molecule is used for MN blood grouping.
Cell invasion
While glycoproteins can help protect the epithelial tissue, especially in the mucosa, it has been shown to contribute to cellular invasion in some cases. Here, the molecule (e.g. HMW1 adhesin in Haemophilus influenzae) promotes attachment of the pathogen to the host cell thus promoting invasion.
In some cases, pathogens have been shown to glycosylate proteins of the host in order to invade the cells of the host. In this case, glycosylation of the host proteins allows the pathogen to attach to the cell and consequently invade the cell.
Return from What are Glycoproteins? to MicrscopeMaster home
References
Alfred Jay Bollet. (1959). Plasma Glycoproteins, Mucoproteins, and Mucopolysaccharides.
Berg JM, Tymoczko JL, and Stryer L. (2002). Carbohydrates Can Be Attached to Proteins to Form Glycoproteins.
Borong Lin, Xue Qing, Jinling Liao, and Kan Zhuo. (2020). Role of Protein Glycosylation in
Host-Pathogen Interaction.
Lodish H, Berk A, and Zipursky SL, et al. (2000). Molecular Cell Biology. 4th edition.
Links
https://basicmedicalkey.com/glycoproteins/
https://www.thoughtco.com/glycoprotein-definition-and-function-4134331
https://www.yourhormones.info/hormones/luteinising-hormone/
Find out how to advertise on MicroscopeMaster!