Sertoli Cells
Function, Location, Histology and Secretion
Overview
Discovered in 1865 by Enrico Sertoli, an Italian scientist, Sertoli cells reside in the seminiferous tubules where they are involved in several functions including the formation of the blood-testis barrier (hemato-testicular barrier) as well as providing nourishment for sperm cells.
Function and Location
As mentioned, Sertoli cells are located in the seminiferous tubules where they originate from the epithelial sex cords. Given that their development is one of the core events in the development of testis, Sertoli cells start to differentiate early on in an organism.
Based on studies involving mice, researchers noticed that early on (in mice embryos that were sexually undifferentiated), the proliferation of coelomic epithelium results in the development of genital ridges at the ventromedial surface of the mesonephroi. These ridges were visible about 11 days postcoitum.
The ridges are responsible for the production of gonads (with the potential of developing into either male or female gonads). Along with some other cells, the gonads contain (somatic cells) primordial germ cells that originate from the epiblasts located in the extraembryonic mesoderm. Somatic cells and primordial germ cells give rise to the gonadal blastema where the sexual cords are formed.
The development of testis is characterized by several important events that include the differentiation of the Pre-SCs (pre-Sertoli cells) as well as the migration of endothelial cells from the neighboring mesonephros. Although pre-SCs cells originate from the epithelial sex cords, studies have shown Sertoli cells to be mesenchymal in nature.
As such, they first transition from mesenchymal to epithelial type of cells before they ultimately differentiate into proper Sertoli cells. The differentiated Sertoli cells make up the epithelium of the seminiferous tubules that envelop the germ cells.
* The number of Sertoli cells have been shown to be directly related to sperm output as well as testicular size - The higher the number of Sertoli cells the high the sperm output.
* In primitive gonads, Sertoli cells are responsible for influencing the first stage of development of embryonic testis. This is achieved by the expression of SRY gene which not only influences development of the male sex of the gonad, but also prohibits the development of internal female genitalia.
* Sertoli cells elongate as an individual approaches puberty and only proliferate during the first year of an individuals' life.
In the male reproductive system, seminiferous tubules (where Sertoli cells reside) are located within the testes. From here, they extend into the efferent ductules (may range from12 to 20 in total) which transmit seminal fluids to the epididymis.
From the epididymis, these fluids are carried through several parts of the reproductive system before ultimately being released into the ejaculatory ducts so that they can be released through the urethra. Therefore, Sertoli cells are well-positioned to contribute to the reproductive system.
Histology
In order to view and study the characteristics of Sertoli cells, it's important to prepare a section of the testicular sample for microscopy.
Requirements
- Specimen
- Sharp razor
- Pair of gloves
- Modified Bouin's solution or
- Helly's solution
- Dehydrating alcohol (ethanol)
- Water
- Burner
- Piccolyte Paraplast
- Oven
- Microtome
- Hematoxylin and Eosin
- Compound microscope
Preparation Procedure
Here, a mouse testis can be used as the specimen. The specimen should be treated with care to avoid causing damage. For instance, only the sharpest razor should be used to cut small a small section to be prepared.
Once the specimen has been obtained, students should make sure to not only put on a pair of gloves when cutting it to obtain small sections but also to try and do this quickly so that the sections can be fixed within the shortest period of time possible.
Fixation
To fix the specimen, modified Bouin's solution (Cleland's solution) or Helly's solution can be used. These solutions have been shown to work much better than formalin which is commonly used to preserve various specimens.
To ensure that the specimen remains in good condition, the fixative is usually prepared while the specimen is being prepared. Once the specimen is read, the small sections/pieces can be gently placed into the fixative.
During fixation, it's usually recommended that you gently place the pieces/sections into the jar (containing the fixative) gently while avoiding disturbing the specimen. For this reason, avoid poking the specimen with the blade or forceps once it has been placed in the fixative.
* One of the biggest advantage of using Cleland's solution is that it causes minimum shrinkage while also allowing for good cellular differentiation following the staining process.
Embedding
The next phase of tissue preparation involves dehydration and embedding. Once the tissue has been fixed (for between 8 and 12 hours), then it has to be dehydrated for several hours. This is a stepwise procedure that involves transferring the tissue from one concentration of the alcohol for a few hours to another.
For instance, the tissue is placed in 30 percent ethanol for between 2 and 4 hours before it's transferred to 50 percent ethanol for 3 to 4 hours. It's placed in 70 percent for 4 to 24, 90 percent ethanol for about 2 hours, 95 percent ethanol for 2 hours, and ultimately absolute ethanol for about 2 hours.
Serial dehydration using an increasing concentration is important in that it ensures that the tissue is properly dehydrated without resulting in significant damage. Here, water removal through dehydration is an important step that prepares the tissue for embedding. Water does not mix with most of the media used for embedding.
Following dehydration, the tissue can then be embedded using 6 percent Piccolyte Paraplast at 60 decrees C. To prepare the embedding material, a mixture of chloroform, Piccolyte and Paraplast are heated for 2 hours at about 56 degrees C in an oven (to evaporate the chloroform).
Once this material cools, the tissue section is passed through three changes of the material, spending an hour in each change so that it is maintained in the material for a total of 3 hours. Ultimately, the tissue is embedded in the material (6 percent Piccolyte Paraplast) at 60 degrees C.
Microtony
Before the tissue sample is stained for microscopy, it has to be cut into thin sections to produce thin slices that can allow light to pass through. To cut the tissue into thin sections, a microtome is used.
For this experiment, the sections can be cut using the AO Spencer microtome at 4u. In a case where the sections are allowed to fall into a water bath at 40 degrees C, they then can be picked using a slice coated with an adhesive (e.g. Mayer's adhesive). To dry the slide, it's placed in a warm oven for about 10 minutes.
The sample is often sealed using a very thin layer of paraffin around the edges to preserve the slide for a long time.
Staining
The last step before mounting involves staining. In histology and histopathology, H and E (Hematoxylin and Eosin) staining is one of the most commonly used techniques for the demonstration of nucleus and cytoplasmic components of a cell.
Staining Procedure
· If the slide is to be viewed under the microscope, then the section has to be deparaffinized. This will involve passing the slide over a burner while avoiding overheating
· Hydrate the specimen by passing the slide through decreasing the concentration of alcohol (starting with 100 percent alcohol)
· Allow the slide to dry and stain with hematoxylin for about 4 minutes
· Gently wash the slide with running water for a few (about 3 minutes) minutes until the section has a blue appearance/color
· Pour 1 percent acid alcohol and then wash the slide with running water
· Dip the slide in an alkaline solution and then water with running water again
· Stain the slide with Eosin for about 10 minutes
· Wash the slide with running water for about 2 minutes
· Dehydrate the slide with increasing concentration of alcohol and then clean using xylene
· Mount on the microscope and record your observation - You can start at 40X magnification and increase to 400X
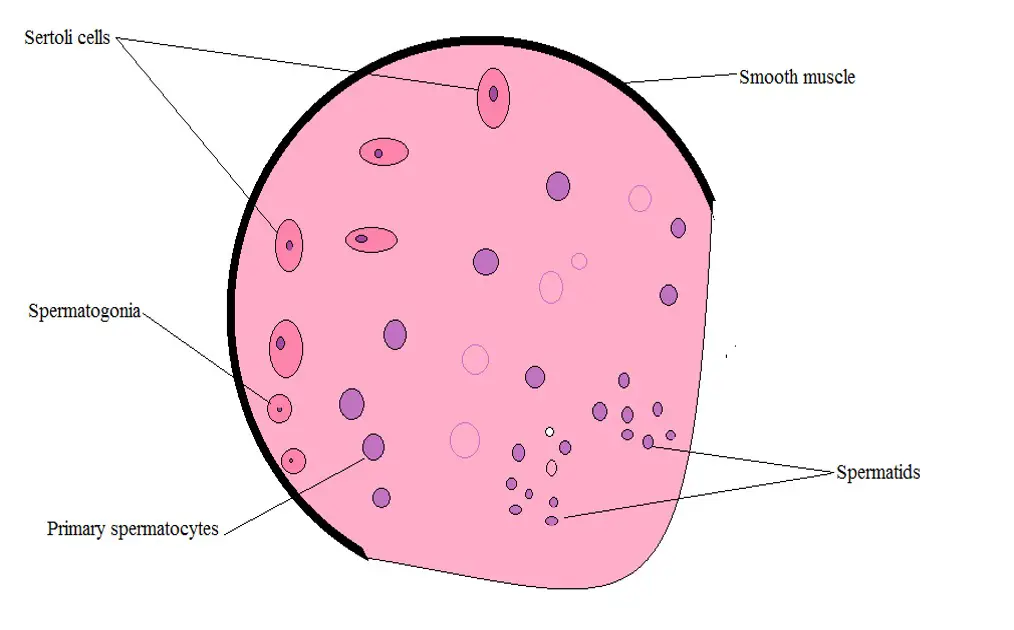
Observation: Structure of Sertoli Cells
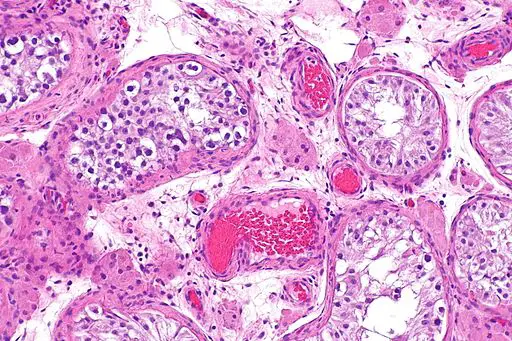
At low magnification, it's difficult to distinguish between Sertoli cells and the surrounding cells. However, as the magnification increases (400X to 1000X), Sertoli cells can be distinguished based on their morphological characteristics.
At high magnification, Sertoli cells appear to be elongated (an oval shape) with a darkly stained nucleus (the nucleus stains dark purple in color while the cytoplasm has a pinkish appearance).
While Sertoli cells were initially suspected to be syncytium, studies that involved the use of electron microscopy allowed for the identification of their cellular membrane as well as junctional complexes thus proving that they are in fact individual cells.
Using the light microscope, on the other hand, one of the most distinguishable organelles of these cells is the nucleus. In addition to being large in size, the nucleus has been shown to be capable of changing shape. This has been attributed to the fact that it's euchromatic and often undergoing transcription.
* The nucleus is surrounded by a nuclear membrane that has been associated with vimentin intermediate filaments.
The nucleolus of these cells, which is tripartite, is also very large and tends to stain more intensely compared to the other parts of the nucleus. In most cells, the nucleus has been shown to reside near the basement membrane of the cell.
Some of the other characteristics associated with this nucleus include:
· They have a deep cleft on the nuclear envelope - Characteristic of mature Sertoli cells
· High density of nuclear pore
* Like many other eukaryotic cells, Sertoli cells contain a number of other membrane-bound organelles. Mitochondria and the rough endoplasmic reticulum, in particular, are abundant in these cells which is an indication that they are involved in high metabolic activities.
* While Sertoli cells have been shown to be very resistant to chemicals (as compared to the other testicular cells), aging of these cells results in changes in the shape of the nucleus, alterations of the endoplasmic reticulum and lysosomes.
While the cells have been shown to be elongated, appearing ovoid in shape, they have also been shown to have branch-like cytoplasmic arms that wrap around the germ cells (involved in germ cell development). These cytoplasmic arms are thin with a width of less than 50nm.
They join at some point to form cup-like regions that interact with the germ cells. These arms are particularly important in that they increase the general surface area of the cells thus allowing them to effectively carry out their functions.
Before interacting with the germ cells, Sertoli cells form tight junctions (ScTj; Sertoli-Sertoli cell junctions) that in turn form the blood-testis barrier, also known as the Sertoli cell barrier. Using electron microscopy, these barriers have been shown to be significantly tight in vivo.
Some of the other components of this barrier include such proteins as actin, clauding and gelson. However, these components have been shown to vary between different organisms.
In all animals, however, the barrier is located near the base of the seminiferous tubule. It's responsible for dividing the epithelium into two main compartments known as the basal compartment and the adluminal compartment.
Some of the cells (spermatogonia and preleptotene) are contained within the basal compartment while others, primary and secondary spermatocytes and spermatids, are contained in the adluminal compartment. Through these barriers, then, the blood-testis barrier serves to isolate the germ cells (located in the adluminal compartment) from both the circulatory and lymphatic systems.
Secretion
Apart from forming the blood-testis barrier, Sertoli cells also secrete a number of molecules associated with the reproductive system.
These include:
Androgen-binding protein - ABP is secreted in the basal and adluminal compartments by Sertoli cells under the influence of Follicle-stimulating hormone (FSH). Although the protein is secreted in these compartments, the majority of it (about 80 percent) is released to the epididymis. Once it's released, androgen-binding protein binds to testosterone to regulate spermatogenesis.
Anti-Müllerian hormone - Also known as Mullerian inhibiting factor, the anti-Mullerian hormone is a glycoprotein that is involved in sexual differentiation in animals. Like ABP, the anti-Mullerian hormone is secreted by Sertoli cells during the fetal stage of development.
Once it's produced, it serves to regress the development of various organs of the female reproductive system including the uterus, the Mullerian ducts as well as the fallopian tubes thereby prohibiting the development of intrinsic female genitalia in males.
Inhibin and activins - Like the other substances, inhibin and activins are also produced by Sertoli cells. However, they are normally produced once an individual enters puberty. Under the influence of FSH, Sertoli cells, along with another group of cells, produce inhibin B which is released into the cycling system.
In the process, it inhibits pituitary production through the endocrine pathway. Activins, on the other hand, promote the biosynthesis and production of FSH as well as other functions in the menstrual cycle in the female reproductive system.
Estrogen - While estrogen has been shown to have a negative impact on the reproductive capacity of male individuals when they are exposed to the hormone before birth, studies have shown immature Sertoli cells to be capable of producing it through their aromatase (enzyme responsible for estrogen production). Over the course of the maturation process, these cells also continue to express estrogen receptor-beta
Take a look at Acrosomes - Definition, Function, Reaction and Exocytosis
Also read about Centrioles - Definition, Function, Structure of Plant/Animal Cells
Return from Sertoli cells to MicroscopeMaster home
References
Dolores D. Mruk and C. Yan Cheng. (2015). The Mammalian Blood-Testis Barrier: Its Biology and Regulation.
Francisco Barrionuevo, Miguel Burgos and Rafael Jiménez. (2011). Origin and function of embryonic Sertoli cells.
Lixin Feng and Yongguang Yang. Origin and Quantitative Control of Sertoli Cells.
Maria Namwanje and Chester W. Brown. (2016). Activins and Inhibins: Roles in Development, Physiology, and Disease.
Petersen C and Söder O. (2006). The Sertoli Cell – A Hormonal Target and ‘Super’ Nurse for Germ Cells That Determines Testicular Size.
Links
https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/sertoli-cell
Find out how to advertise on MicroscopeMaster!