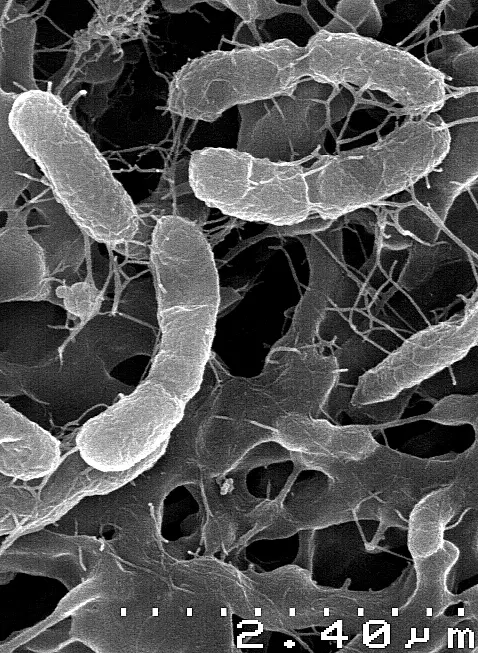
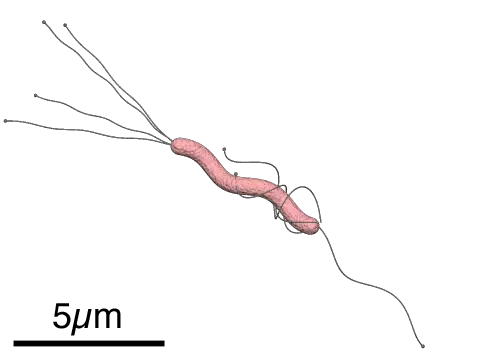
Chemosynthetic Bacteria
Definition, Examples, Pathways and Processes
Definition: What are Chemosynthetic Bacteria?
Essentially, chemosynthetic bacteria include a group of autotrophic bacteria that use chemical energy to produce their own food. Like photosynthetic bacteria, chemosynthetic bacteria need a carbon source (e.g. carbon dioxide) as well as an energy source in order to manufacture their own food.
For the most part, these bacteria are aerobic and therefore rely on oxygen to complete this process successfully. However, some species (e.g. Sulfuricurvum kujiense) have been associated with anaerobic chemosynthesis.
Because of their ability to manufacture their own food using chemical energy, these organisms are able to survive in a variety of habitats/environments including harsh environments with extreme conditions as free-living organisms or in association with other organisms through symbiosis with other organisms.
* Unlike photosynthesis which is common in eukaryotic organisms and cyanobacteria, chemosynthetic reactions are mostly carried out by prokaryotic microorganisms (particularly bacteria and archaea)
Examples of chemosynthetic bacteria include:
- Venenivibrio stagnispumantis
- Beggiatoa
- T. neapolitanus
- T. novellus
- ferrooxidans
Types of Chemosynthetic Bacteria
As mentioned, chemosynthesis allows different types of bacteria (chemosynthetic bacteria) to survive without relying on light energy or other organisms for food.
Here, the energy used to manufacture food materials is derived from a variety of inorganic chemicals and thus different chemical reactions. For this reason, there are different types of chemosynthetic bacteria based on the type of compounds they use as an energy source.
* Some chemosynthetic bacteria live in sunny environments and are therefore exposed to sunlight. However, they do not rely on sunlight as a source of energy
Sulfur bacteria - These bacteria (e.g. Paracoccus) oxidize such sulfur compounds as hydrogen sulfide (sulfides) thiosulfates and inorganic sulfur etc. Depending on the organism, or the type of sulfur compound being used, the oxidation process takes place in several stages.
In some of the organisms, for instance, inorganic sulfur will be stored until they are required for use.
Nitrogen bacteria - Divided into three groups that include nitrifying bacteria, denitrifying bacteria, and nitrogen-fixing bacteria. In the case of nitrifying bacteria, ammonia is first oxidized to hydroxylamine in the cytoplasm (by ammonium monooxygenase).
The hydroxylamine is then oxidized to produce nitrite in the periplasm by hydroxylamine oxidoreductase. This process produces a proton (one proton for each molecule of ammonium). As compared to nitrifying bacteria, denitrifying bacteria oxidize nitrate compounds as a source of energy.
Methanobacteria/methane bacteria - Although some scientists have suggested that some bacteria use methane as a source of energy for chemosynthesis, this is particularly common among chemosynthetic archaebacteria.
Hydrogen bacteria - Such bacteria as Hydrogenovibrio marinus and Helicobacter pylori oxidize hydrogen as a source of energy under microaerophilic conditions.
For the most part, these bacteria have been shown to be anaerobic and therefore thrive in areas with very little to no oxygen. This is largely due to the fact that the enzyme used for oxidation purposes (Hydrogenase) functions effectively in anaerobic conditions.
Iron bacteria - Acidithiobacillus ferrooxidans and Leptospirillum ferrooxidans are some of the bacteria that oxidize iron. This process has been shown to occur under different conditioned depending on the organism (e.g. low pH and oxic-anoxic).
During chemosynthesis, chemosynthetic bacteria, being non-photosynthetic, have to rely on energy produced by oxidation of these compounds (inorganic) in order to manufacture food (sugars) while nitrogen-fixing bacteria convert nitrogen gas into nitrate. All these processes serve to produce a proton used in carbon dioxide fixation.
Normally, these reactions occur in the cytoplasm in the presence of membrane-bound respiratory enzymes. For instance, in the case of hydrogen oxidation, group 1 NiFe hydrogenases, found in the cytoplasm, catalyze the reaction to produce 2 electrons and protons (hydrogen with a positive charge) from a hydrogen molecule (H2 <>2H+ and 2e-). These electrons are then channeled to the quinone pool in the electron transport chain.
In the case of hydrogen sulfide, the compound undergoes oxidation to release electrons and hydrogen ions (referred to as protons given that they are separated from the compound and electrons and gain a positive charge). The products of this reaction are therefore sulfur, electrons as well as protons. Electrons and protons then enter the electron transport chain (at the membrane).
As electrons enter this chain, protons are pumped out of the cell. Electrons, on the other hand, are accepted by oxygen and attract the protons (hydrogen ions) thereby forming water molecules. Through an enzyme known as ATP synthase, protons that had been previously pumped out of the cell are channeled back to the cell with their energy (kinetic energy) is stored as ATP and is used for sugar synthesis.
Carbon Assimilation in Chemosynthetic Bacteria (fixation)
Depending on the type of bacteria, their habitat, and carbon source, there are a number of metabolic pathways used for fixation.
Some of the most common pathways include:
Calvin-Benson Cycle - In this cycle, the enzyme RuBisCo (ribulose 1, 5-bisphosphate carboxylase/oxygenase) facilitates the addition of molecular carbon dioxide to ribulose 1, 5-bisphosphate. This process generates a six-carbon compound that is, in turn, converted into two molecules of 3-PGA (3-phosphoglycerate). This process is referred to as carbon fixation given that it involves the conversion of carbon dioxide into organic molecules.
Through the energy stored in ATP and NADPH (generated through the oxidation process), the carbon compound (3-PGA) is again converted into another carbon compound to form G3P (Glyceraldehyde 3-phosphate) in the reduction phase.
As one of these molecules leaves the Calvin chain (to form the carbohydrate molecule/sugar), the other is involved in the generation of RuBP.
Krebs Reverse Cycle - As compared to the Calvin cycle, carbon fixation in Krebs Reverse Cycle results in the production of pyruvate. Also known as the Reductive Tricarboxylic Acid Cycle, this cycle starts with the fixation of two molecules of carbon dioxide. It results in the production of acetyl coenzyme A (acetyl-CoA) that is in turn reductively carboxylated to produce pyruvate.
The pyruvate produced through the process is then used for the synthesis of the organic cell materials.
Some of the other processes used by these bacteria include:
· 3-Hydroxypropionate Bicycle - Also known as the 3-hydroxypropionate cycle, this pathway fixes carbon dioxide to form Malyl-CoA in the presence of acetyl-CoA and propionyl-CoA carboxylases. This is then split to produce acetyl-CoA and glyoxylate. Ultimately, the pathway results in the production of pyruvate which is used for synthesizing various organic materials required by the cell.
· Reductive Acetyl-CoA Pathway - In this pathway, two molecules of carbon dioxide are fixed to form acetyl-CoA. Typically, hydrogen acts as the electron donor in this reaction with Carbon Dioxide being the electron acceptor.
· Dicarboxylate/4-Hydroxybutyrate Cycle - This cycle is common among bacteria found in anaerobic and microaerobic habitats (e.g. Desulfurococcales). Like the 3-hydroxypropionate/4-hydroxybutyrate cycle, this cycle converts cetyl-CoA and two molecules of carbon into succinyl-coenzyme (CoA). Some of the enzymes involved in this cycle include pyruvate synthase and phosphoenolpyruvate (PEP) carboxylase.
Importance of Chemosynthetic Bacteria
Essentially, chemosynthesis refers to the process through which chemosynthetic bacteria process food using chemical energy. Therefore, compared to photosynthesis, these organisms are not dependent on light energy for production. This makes them important primary producers in various habitats that contain such oxidants as nitrates and sulfates.
In deep-sea vent ecosystems, for instance, the absence of sunlight means that photosynthesis cannot take place. Because of the ability of some bacteria to manufacture food through chemosynthesis, they play an important role as producers in this ecosystem.
This behavior has also been shown to benefit other organisms through a symbiotic relationship. For instance, in various environments, nitrogen-fixing bacteria have been shown to form symbiotic relationships that benefit a variety of organisms (algae, diatoms, legumes, sponges, etc). Here, they are able to convert nitrogen (abundant in nature) into useable forms.
Here, these bacteria can catalyze atmospheric nitrogen to produce ammonia (using an enzyme known as nitrogenase) which is then used by plants for the synthesis of nitrogenous biomolecules.
One of the other symbiotic relationships that have received significant attention is between tubeworms (Riftia pachyptila) and chemosynthetic bacteria in hydrothermal vents. In this environment, water temperatures are extremely high due to geothermal heat. Moreover, these worms live at the seafloor (environment lacking light energy).
Despite the unfavorable conditions in this environment (extremely high temperatures and lack of light), the availability of hydrogen sulfide allows bacteria to carry out chemosynthesis.
Using a highly vascularized gill-like plume, the worm is able to take in dissolved carbon dioxide, oxygen, and hydrogen sulfide (the hemoglobin of these organisms are capable of binding oxygen and sulfides). They are then transported to specialized cells known as bacteriocytes where chemosynthetic bacteria reside.
Using the sulfide and oxygen, the bacteria produce energy (ATP) that is then used to convert carbon dioxide into sugars. These sugars are then used by the mollusk as a source of food.
Such symbiotic relationships have also been identified with:
- Solemyid and lucinid bivalves
- Achinoids
- Ciliate protists
- Marine sponges
- Mussels
Some of the characteristics that have been associated with the symbiont (chemosynthetic bacteria) include:
· Have a Gram-negative envelope
· Vary in shape from small coccoid endosymbionts of about 0.25um in diameter to relatively large (about 10um in length) rod-shaped chemotrophic bacteria
· Depending on the species, they may be endosymbionts or simply attach to the body surface of the hosts
Return from Chemosynthetic Bacteria to MicroscopeMaster home
References
Colleen M. Cavanaugh, Zoe P. Mckiness, Irene L.G. Newton and Frank J. Stewart. (2006). Marine Chemosynthetic Symbioses.
H. W . J annasch. (1985). The chem osynthetic support of life and the microbial diversity at deep-sea hydrotherm al vents.
Jennifer J. Wernegreen. (2013). Endosymbiosis.
Zoran Minic andPremila D. Thongbam. (2011). The Biological Deep Sea Hydrothermal Vent as a Model to Study Carbon Dioxide Capturing Enzymes.
Links
https://ocw.mit.edu/high-school/biology/exam-prep/cellular-energetics/photosynthesis/chemosynthesis/
Find out how to advertise on MicroscopeMaster!