Bacterial Transformation
Gene Transfer, Heat Shock, Vs Transduction
Gene Transfer
Essentially, gene transfer in bacterial transformation refers to the movement or transfer of copies of genes between cells of given organisms. Generally, gene transfer has been divided into two main types including horizontal and vertical gene transfer.
In vertical gene transfer, genes are passed down from the parent to the offspring through sexual or asexual means.
Horizontal gene transfer, also known as lateral gene transfer, refers to the movement or transfer of genetic material between cells of given organisms through means other than vertical transmission.
There are three main types of horizontal gene transfer which include transduction, conjugation, and transformation. In nature, horizontal gene transfer occurs naturally where the recipient cell receives foreign genes through contact with other cells, contact with an agent carrying the foreign genetic material, or when the cell simply takes up genetic material in their surroundings.
As well, special chemical and mechanical treatments can be used in the laboratory for the purposes of introducing the desired genetic material into the cell of interest. A good example of this involves subjecting cells (e.g. bacterial cells) to heat shock in order to form pores on the surface of the cells through which the genetic material can enter.
Following entry, the genetic material is then integrated into the chromosomal DNA of the recipient cell where it can then express given traits (e.g. desired proteins , etc).
Heat Shock Bacterial Transformation
Bacterial transformation refers to a horizontal gene transfer process where bacteria take up foreign genetic material (not their own) from their surroundings. Unlike some of the other types of horizontal gene transfer, bacterial cells that undergo transformation only require persistent naked DNA for this process to occur.
While this process can occur naturally (natural transformation), special techniques (artificial transformation) can be used in the laboratory to introduce desired genetic material into given recipient cells for the purposes of replicating sequences of interest for analysis etc. One of these techniques is known as heat shock transformation.
* Of the three types of horizontal gene transfer (transformation, transduction, and conjugation), bacterial transformation was the first mechanism to be described - It was described in 1928 by Fred Griffith, a British bacteriologist.
Some of the main applications of heat shock bacterial transformation include:
- Cloning purposes
- Studies of DNA linkages
- Reproduction of cDNA (complementary DNA) libraries
- For the purposes of reproducing DNA copies for studies
- To express large quantities of proteins and enzymes
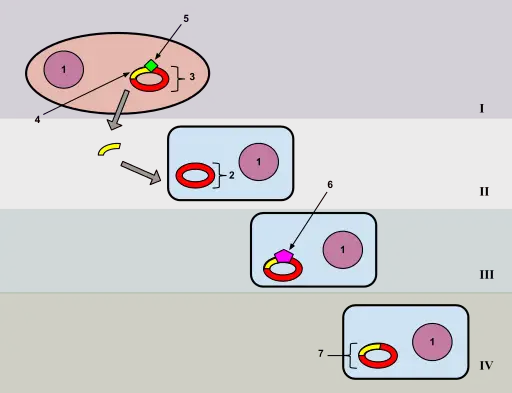
Essentially, heat shock bacterial transformation is centered on exposing bacterial cells (e.g. E. coli) to a heat shock. This refers to a sudden or rapid increase in temperature resulting in pore formation through which the DNA material (e.g. plasmids) can enter the cell. The cells are then allowed to heal and resume their normal life cycle (cell cycle).
The following are some of the material and equipment required for heat shock bacterial transformation:
- Ice
- Microcentrifuge tubes (2ml microcentrifuge tubes can be used)
- Petri dishes
- Heating block or water bath
- Incubator
- Bacteria (e.g. E. coli)
- DNA of interest (e.g. supercoiled plasmid)
- Volume pipette
Protocol of Heat Shock Bacterial Transformation
Before taking any step, it's always important to ensure that all the equipment/material used are clean and properly sterilized to avoid contamination. Specific bacteria are used for the transformation process so one needs to ensure that there is no contamination for better results.
1/ Using a volume pipette, about 20ul of the bacterial cells (in solution) are transferred into the 2ml tubes and are placed in the ice container. Before taking this step, it's important to ensure that the cells remain frozen until they are ready to use.
In the event that they thaw too soon, then the transformation efficiency would be affected. Following the transfer of cells into the 2ml tube, they still need to be kept in the ice container in preparation for heat shock.
2/ To the 20ul of cells, about half a microliter of the plasmid (or appropriate amount of the DNA to be used) is added. For some of the kits used, the plasmids come in pure form and are therefore highly concentrated. As a result, a very small amount is added.
As is the case with the bacterial cells, a volume pipette is used to collect the right amount of the plasmid and introduce them into the 2ml tube containing the cells - Given that this step involves introducing a very small amount of the plasmid (or DNA) it's important to ensure that they are introduced directly into the cells (and not only sides of the tube etc) to avoid any wastage.
3/ The two can then be mixed by carefully tapping/flicking the lower part of the tube and again placed in an ice container in a tube rack for 10 to 20 minutes. This allows some time for the plasmid or DNA to stick or bind to the cell surface. Moreover, it allows the bacterial cells to remain cool before they are subjected to heat shock.
4/ The next step involves performing heat shock at between 37 and 42 degrees C for about 30 seconds. Here, the tube or tubes, containing the bacterial cells and plasmid or DNA, are placed in the heating block (or water bath) for 30 seconds at about 42 degrees C.
Although performing heat shock basically involves subjecting the cells to a sudden increase in temperature, it's worth noting for such cells as E. coli, this action activates the expression of heat shock genes that allow the bacteria to survive. Therefore, they are not destroyed at this relatively high temperature.
As a result of the heat shock, studies have shown that two important events take place. These include; the release of lipids resulting in pore formation of the bacterial cell as well as lowering membrane potential. These two events allow entry of plasmid/DNA into the cell through both the outer and inner membranes.
5/ Once the cells are subjected to heat shock for about 30 seconds, they are again placed in the ice container (using a tube rack) so that they can recover for about 2 minutes.
For further recovery, bacterial cells have to be grown in the appropriate media. Here, one of the most commonly used media is the LB medium or LB broth (Lysogeny broth). Two minutes after being placed in ice, 200ul of the medium is added to the tube containing the bacterial cells using a volume pipette. This gives a ratio of 10:1 (ten parts of the medium and 1 part of the bacterial cells).
The contents in the tube can be mixed by carefully tapping the bottom part of the tube. For this step, Super Optimal Broth (SOC media) can also be used for recovery.
6/ Once the medium has been added, the tube has to be incubated at about 37 degrees C for 15 minutes to one hour. This step should be carried out in a shaking incubator. This provides favorable conditions for the cells to continue recovering.
In a case where plasmids were used, this step has also been shown to allow cell growth as well as the production of antibiotic resistance proteins (expressed from the genes of the introduced DNA/plasmid) which in turn ensures that the bacteria continue to grow when they are grown in agar containing an antibiotic.
7/ Following this initial incubation, the cells are again incubated in the appropriate agar plate containing an antibiotic. This not only allows for the formation of colonies but also helps determine whether the desired traits were expressed.
Here, one may prepare two or more plates with different quantities of the competent cells (including a control plate) to compare the results. For instance, 20ul of cells may be introduced into one of the plates and 200ul added to another plate (consisting of LB agar).
As with some of the steps above, a volume pipette is used to collect and introduce the cells into the agar plates. Moreover, glass beads can also be used to ensure that the cells are evenly distributed on the plate.
* For the control, the same quantities of cells that have not been transformed can be added into two other agar plates.
* In this step, the plates used also contain antibiotic (in the event that the aim of the study is to determine whether antibiotic genes were expressed).
8/ Lastly, the plates are incubated overnight at 37 degrees C to grow and produce colonies. After 15 to 24 hours of growth, cells that were transformed with plasmids grow and colonies can be seen given that the foreign genes allow the bacteria to express antibiotic resistance proteins.
However, for the control cells that were not transformed, colonies are not formed as the antibiotic kills the cells and prevents them from growing. These results are indications that the plasmids were taken in by the cells and produced the desired characteristics.
For other types of bacteria, the same procedure can be used and desired characteristics observed at the end of the experiment.
Principle
As mentioned, the heat shock process results in the release of lipids resulting in the formation of pores on the cell surface of bacteria. This allows the DNA/plasmid to cross the outermost membrane.
Secondly, membrane potential is lowered thus allowing the DNA/plasmid to cross the inner membrane of the bacteria and thus gain entry into the cell.
By decreasing the membrane potential, negativity of the cells inside potential is reduced. This is also important in that it influences the movement of DNA, which is negatively charged, into the cell. This is one of the main reasons the cells have to be kept cool in ice during preparation. Once they are subjected to heat, the temperature changes rapidly.
Once the cells take in the DNA/plasmid, the cells have to be placed in the ice container again for recovery as mentioned in the protocol. Also known as cold shock, this step has been shown to raise the membrane potential and is therefore important in that it returns the membrane to its original state.
Here, it's also worth noting that for the most part, DNA is hydrophilic (water-loving) while the membrane largely consists of a lipid bilayer. For this reason, DNA and plasmids cannot easily pass through it. It is necessary to form relatively large pores through which these materials can be introduced into the cells.
Following the entry of genetic material into the recipient bacterial cell, it's expected that the bacterial cells will start exhibiting given characteristics related to associated genes (genes introduced into the cells).
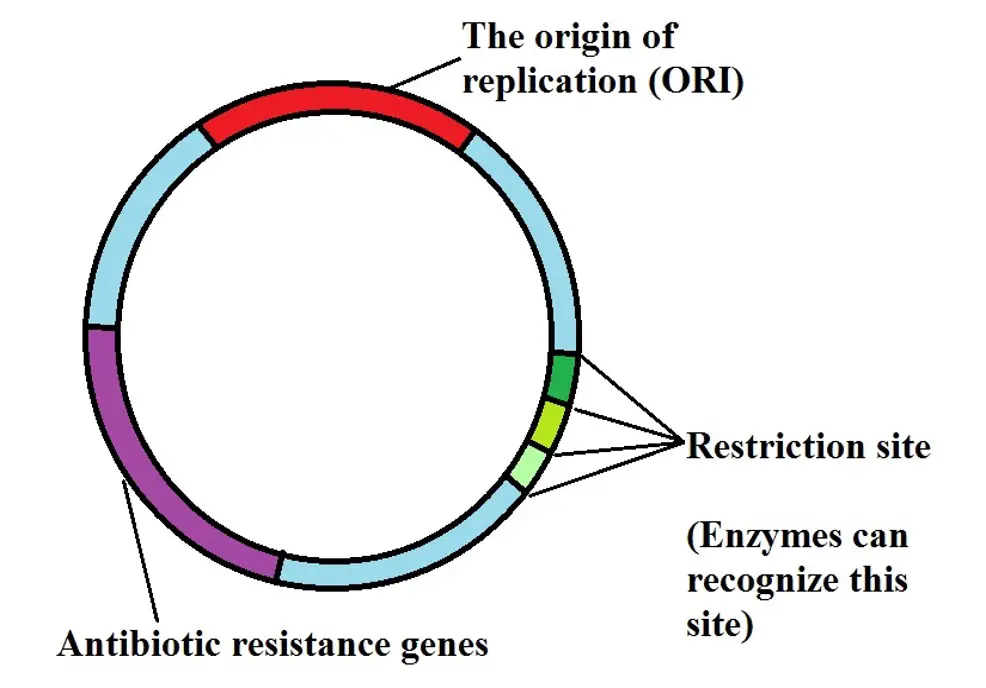
Given that plasmids are distinct from the chromosomal DNA of the cell (plasmids are extrachromosomal DNA molecules), they are not integrated into the chromosomal DNA of the organism. Rather, they are capable of self-replication and carry genes that can be expressed to confer given characteristics to the cell.
One of the other biggest advantages of plasmids is that they can easily be engineered. This means that they are designed to have unique sites where special genes of interest can be inserted (at the restriction sites).
Here, restriction enzymes (which act as molecular scissors) are used to identify these sites and cut out the genes already present so that new genes can be introduced. Generally, expression of plasmid genes occurs when the regulatory elements bind to the promoter region which in turn "green lights the transcription process".
During this process, the RNA polymerase binds to the promoter and moves along the strand to create a new strand of mRNA which is the process through which the gene is expressed. The mRNA is then transcribed to produce products (e.g. proteins) that benefit the cell.
Diagrammatic representation of the main parts of a plasmid:
* When the antibiotic genes or genes at the restriction site are expressed, the bacteria develop characteristics associated with these genes.
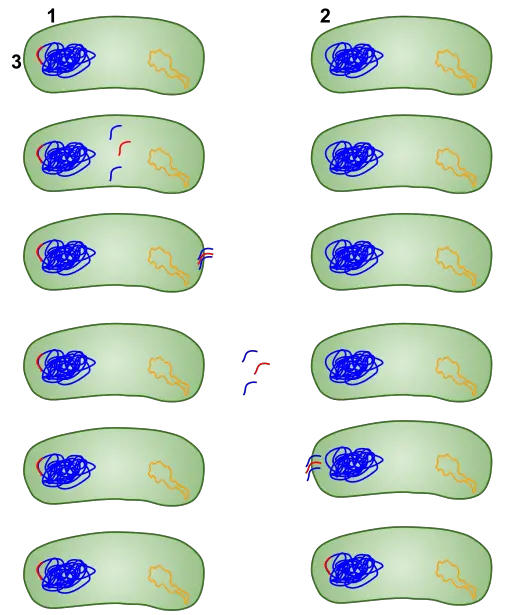
Unlike plasmids that are introduced into the bacterial cells and start to replicate with specific genes being expressed, DNA pieces introduced into the cell through the same technique (heat shock transformation) have to be integrated into the bacterial chromosomal DNA.
Here, studies have shown that for this integration to take place, then the foreign DNA fragment must have between 25 and 200bp in length that is similar to the genome of the recipient bacterial cell. This not only allows for strand exchange, but also for DNA pairing during integration.
While some of the foreign DNA fragments are integrated, some remain in the cytoplasm and are therefore degraded by enzymes. Following a successful integration of the foreign DNA into the chromosomal DNA of the bacteria, the replication process ensures that the new genes introduced continue to be reproduced.
As is the case with foreign plasmid, this process has also been shown to result in new characteristics as new genes introduced produce new characteristics when they are expressed. This has also been associated with mutation in bacteria due to changes in the genetic material of the organism.
Bacterial Transformation Vs Transduction
In bacteria, transformation and transduction are means of gene transfer (horizontal gene transfer). Through both of these mechanisms, the recipient bacterial cell acquires foreign genetic material that ultimately becomes part of their DNA and introduces new genetic characteristics.
Here, as mentioned, it's also worth noting that the two are types of horizontal gene transfer which means that genetic material is transferred from one cell which acts as the donor to another cell that acts as the recipient. This is different from vertical transfer where genes are passed down from the parent cell to the offspring.
While bacteria do not necessarily reproduce sexually, this is sometimes viewed as a form of sexual reproduction given that the process results in genetic diversity of both the parent cells and their offspring.
While bacterial transformation and transduction have a number of similarities, the two are different because the processes involve different mechanisms. One of the main differences between the two is with regard to the manner in which foreign genetic material is obtained by the donor cell.
In natural bacterial transformation, the recipient bacterial cell takes up naked DNA in their surroundings. Whenever bacteria die (e.g. through lysis), different parts of the cell are released into the immediate surroundings. Here, naked DNA has been shown to stick/attach to other living bacterial cells with limited DNA fragments gaining entry into the cell.
Transduction requires the presence of a transporting agent (bacteriophage) to carry genetic material from the donor cell to the recipient. Whereas genetic material is simply taken up through the membrane in transformation, it is introduced into the recipient cell by the bacteriophage in transduction.
Given that transduction involves the entry of some viral DNA, this may result in the destruction of bacterial chromosomal DNA in the event that the phages produce DNA degrading enzymes. Therefore, while the phages aid in introducing DNA from foreign bacteria into the recipient bacterial cell, this may be to the detriment of the cell.
In transformation, this is less likely to occur as foreign bacterial DNA is directly taken up by the recipient bacterial cell. The foreign genetic material in this case is either likely to be integrated or degraded in the cytoplasm.
Return to Recombinant DNA Technology
Return to Bacteria Conjugation
Return to Bacteria under a Microscope, Eubacteria
Return to Bacteria - Size, Shape and Arrangement
Return from Bacterial Transformation to MicroscopeMaster home
References
Christopher M. Thomas and Kaare M. Nielsen. (2005). Mechanisms of, and Barriers to, Horizontal Gene Transfer Between Bacteria.
Christof Heppa and Berenike Maier. (2016). Kinetics of DNA uptake during transformation provide evidence for a translocation ratchet mechanism.
Mohammed F. Al Marjani Al-Mustansiriya. (2015). Bacterial Genetics. Edited by Mohammed Faraj Al-Marjani Department of Biology College of Science Al - Mustansiriyah University.
P M Bennett. (2008). Plasmid encoded antibiotic resistance: acquisition and transfer of antibiotic resistance genes in bacteria.
Subrata Panja,Pulakesh Aich,Bimal Jana and Dr Tarakdas Basu. (2007). How does plasmid DNA penetrate cell membranes in artificial transformation process of Escherichia coli?
Links
https://www.mybiosource.com/learn/testing-procedures/cacl2-transformation-technique/
Find out how to advertise on MicroscopeMaster!