How does Chromatography work?
- Types, Uses and Applications -
Essentially, chromatography is a versatile method through which different kinds of chemical mixtures of substance can be separated. Here, the word versatile is included in the definition because there are a number of techniques that can be used to separate a chemical substance into its individual components. Various chemical substances are made up of a number of individual components.
For instance, such substances as food colorings, plants dyes and inks contain several components. Using chromatograph techniques, it becomes possible to separate these components depending on the needs of the technician. Here, I will focus on different types of chromatography, their uses and application in microscopy.
Basics
Basically, separation of compounds is achieved by dissolving the mixture in a mobile phase and passing it over a stationary phase. Here, the molecules that interact more strongly with the stationary phase, with which they have greater affinity move slowly through the resin while those that have a weak interaction move through it much faster.
Ultimately, this results in the components within the substance being separated. Chromatography may be used to either analyze or purify molecules of a given substance. Therefore, there are two major categories of chromatography: Analytical chromatography and Preparative chromatography.
Preparative Chromatography and Analytical Chromatography
Preparative chromatography is largely concerned with the isolation and purification of given molecules within a substance. It's therefore largely used for various purification purposes as is the case with laboratory-scale protein purification in biochemical characterization in the biopharmaceutical industry.
Analytical chromatography is different from preparative chromatography in that the separation of molecules in a substance is for the purposes of identifying and quantifying the components of the substance. It therefore serves as the best technique for observing what happens to a substrate in a chemical reaction or testing the presence of a given substance or component of interest in a given mixture among others.
Before looking at the different types/techniques, it is important to know some of the terms used.
Mobile phase - Also referred to as the carrier, the mobile phase refers to the solvent that moves through the column
The stationary phase - The stationary phase is also often referred to as the adsorbent and is the substance that remains fixed in the column
Eluent and Eluate - The eluent refers to the fluid that enters the column, the eluate is the fluid that collects in flasks after exiting the column
Analyte - This is the mixture that has been separated into individual components for analysis.
In chromatography (normal-phase) the stationary phase is always hydrophilic in nature, meaning that it is polar while the mobile phase is non-polar which means that it is hydrophilic. In some cases, technicians will use reverse-phase where the stationary phase is non-polar while the mobile phase is polar.
Although there are different types of chromatography that vary depending on the type of stationary and mobile phase used, the basic principle is the same. That is, differential affinities of different components in the substance towards the stationary and mobile phases causes differential separation of the components.
The following are some of the most common techniques:
Paper Chromatography
This is one of the most common types. Paper chromatography is an analytical method used for the purposes of separating colored constituents in a substance. With paper chromatography, the stationary phase is typically solid cellulose while the mobile phase is liquid.
With paper chromatography, the paper (cellulose paper) is typically suspended in a container that contains a shallow layer of the solvent (or in some cases a mixture of solvents). A line with spots is made near the bottom of the paper (spots with the substance) and the solvent has to be just below this line. As the solvent slowly travels up the paper, different constituents of the substance (in the spots) also travel up the paper at different rates as they separate. After the separation, the different components of the compound can be seen directly on the cellulose
The distance travelled relative to the solvent is referred to as the Rf value. For different compounds, this may be worked out using the following formula
Rf = distance travelled by the compound/distance travelled by the solvent
This is also used to identify the type of components.
Some of the main uses of paper chromatography include:
- Qualitative method to identify components of a mixture
- Crime scene investigation and DNA/RNA sequencing
- In analytical chemistry to identify and separate coloured mixtures.
- In scientific studies to identify unknown organic and inorganic compounds from a mixture.
Thin Layer Chromatography
This technique is a type of planar chromatography where the stationary phase is on a flat plate while the mobile phase travels through the stationary phase by capillary action. Thin layer chromatography is also a qualitative analytical chromatography method that is commonly used for the purposes of separating nonvolatile molecules.
This technique uses solid silica or alumina for the solid phase and a mobile liquid phase (such as cyan). The substance of interest is separated on the basis of the polarity of the molecules. Unlike paper chromatography, glass is coated with a thin layer of silica in thin layer chromatography on which the compound is spotted for separation. As is the case with paper chromatography, the solvent travels up the plate through capillary action along with the analyte as its components are separated.
Uses of TLC
Thin layer chromatography can be used for:
- Determine the number of components in a given mixture
- To monitor reaction progress
- To compare compounds
- To determine the effectiveness of a separation achieved on a column
- To determine the appropriate solvent for column chromatography
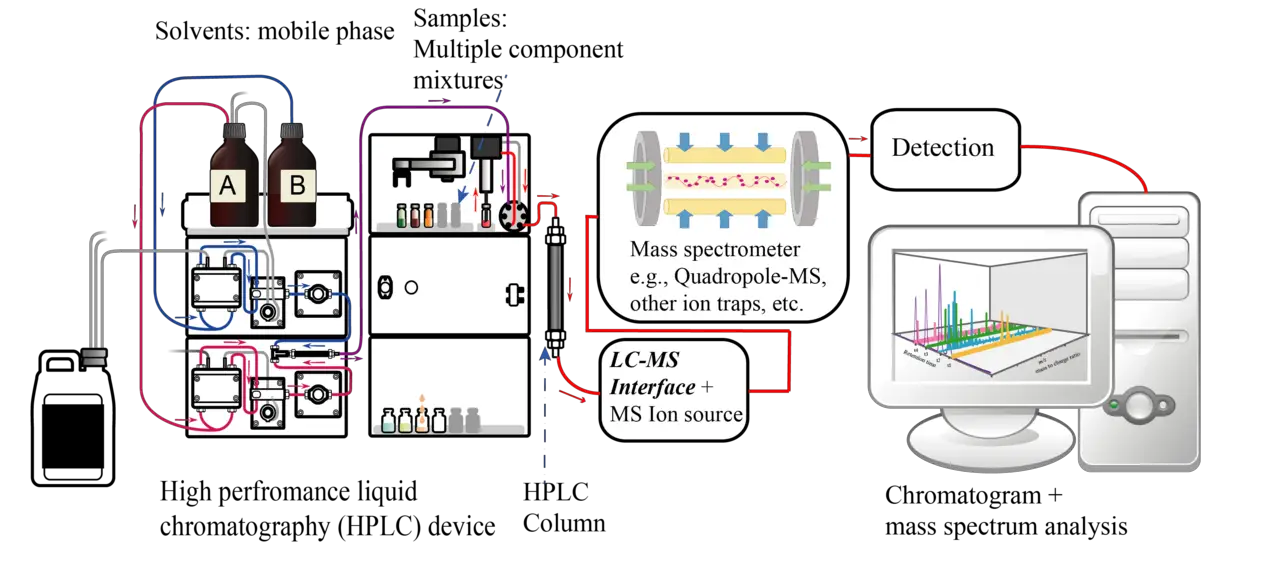
Liquid Column Chromatography
Liquid column chromatography also uses solid silica or alumina for the stationary phase and a liquid phase. Unlike TLC, liquid column chromatography uses microporous beads of silica. Liquid chromatography may be used for either analytical or preparative applications.
As the mobile (liquid) phase travels through the column, components in the mobile phase interact with the solid phase at varying degrees as the molecules of interest get separated on the basis of their varying physiochemical interactions with the mobile and stationary phases.
During the separation process, the small molecules get trapped in the pores of the stationary phase while the larger ones flow through the gaps between the beads and have very small retention rates.
With this technique, there is no chemical or physical interaction between the analyte and the stationary phase. Some of the main uses of this technique include purifying individual chemical compounds from a mixture of compounds and in preparative applications.
Ion-Exchange Chromatography
This technique is one of the most popular techniques used for the purification of proteins and other charged molecules. It uses cationic/anionic resign for the solid phase and a liquid. The separation here is based on the ionic charges of the molecules.
For the cation exchange chromatography, the positively charged molecules are usually attracted to the negatively charged solid support while in anion exchange chromatography, the negatively charged molecules are usually attracted to the positively charged solid support.
In this system, the mobile phase is generally a low medium conductivity solution. Adsorption of molecules to the solid support is driven by anionic interaction between the oppositely charged ionic groups in the sample molecule and in the ligand on the support. Here, the strength of the interaction is largely determined by the number of location of the charges on the molecule and the functional group.
Overall, the molecules that process opposite charge as the resin bind tightly to the resin while those that have the same charge as the resin flow through the column and elute out first.
Because of its ability to separate molecules on the basis of their total charge, Ion Exchange Chromatography allows for the separation of similar types of molecules that would otherwise be difficult to separate using the other techniques.
For this reason, it is commonly used to separate such biological molecules as:
- Proteins
- Amino acids
- Nucleotides
This technique is often used for:
- Separation of vitamins and other biological compounds
- Water purification
- To determine the base competition of nucleic acids
- For analysis of amino acids
Affinity Chromatography
Affinity chromatography is a type of chromatograph that is used to separate given compounds on the basis of specific binding interaction between immobilized ligand and the component in question. For this technique, agarose or porous glass beads are used as the solid phase where separation is based on the binding affinity of the analyte molecule to the molecule immobilized on the stationary phase.
In case the molecule is a substrate for the enzyme, then it binds tightly onto the enzyme while the unbound analyte passes through in the mobile phase, eluting out of the column and leaving the substrate bound to the enzyme. Using the right solvent, it is also possible to detach the substrate from the stationary phase and elute it out of the column.
Some of the primary uses of Affinity chromatography include:
- The purification and concentration of substances from a mixture in to a buffering solution
- To reduce the amount of substance in a given mixture
- To purify and concentrate enzyme solutions
- For discerning the type of biological compounds that bind a given substrate
Gas Chromatography
Gas chromatography is the technique that entails the vaporization and injection of the sample in to the head of a chromatographic column, which is then transported through the column by the flow of the gaseous mobile phase. Here, the column contains a liquid stationary phase that is adsorbed on to the surface of an inert solid. Inert gases like argon or helium are preferred for this technique given that they are not reactive and would therefore not react with the sample.
This technique works on the basis of the boiling point of the molecules. Here, the sample has to be volatilized which results in the molecule with the lowest boiling point coming out of the column first. On the other hand, the molecule with the highest boiling point comes out last.
Gas chromatography is typically used in:
- Analysis of various body fluids and secretions containing large amounts of organic volatiles
- Analysis of air samples
- To determine the components of certain mixtures using retention time and abundance samples in pharmaceuticals
Chromatography and Microscopy
Recently, new techniques are being developed to combine chromatic techniques with microscopy. While some of these processes may be complicated, they are being designed to help scientists and technicians have a better understanding of various material. One of the best examples of this is gas chromatic-mass spectrometry and dispersion-relation fluorescence spectroscopy.
Fluorescence microscopy has for a long time been used for studying labeled structures like cells. With new advancements and developments, this technique is now being used to supply greater spatial and temporal resolutions.
Whereas gas chromatography-mass spectrometry (GC-MS) is typically used for the purposes of identifying the different substances in a sample, fluorescence spectrometry is used to tag given molecules with a flourophore whose movement helps create spontaneous fluorescence intensity fluctuations, which can be examined in order to measure governing mass transport dynamics. This technique has been increasingly used to examine the molecular transport and diffusion coefficients at fixed spatial scales.
With current advancements in fluorescent protein and synthetic flourophore technology, fluorescent live-cell imaging is also expected to play an important role to examine the localization, assembly and the role of various components in such systems as the secretion system. Therefore, microscopy, and particularly fluorescence microscopy can play a very important role in chromatography where it can be used to not only observe various components of given compounds, but also compare these components during analysis.
In general chromatography allows scientists and other technicians to separate and analyze the various components of given compounds. By employing microscopy techniques here, the analysis process is further enhanced given that scientists and technician will be well able to compare the different samples of these components for better analysis.
Related Articles...
- Cell Biology Research
- Blood Microscopy, Sputum Microscopy
- Surgical
- Immunohistochemistry - in cancer research
- Histochemistry
- Urine Analysis, Hematuria
Pathology
Return from Chromatography to MicroscopeMaster Home
References
Thurman, E. M.; Ferrer, Imma (2003). Liquid chromatography/mass spectrometry, MS/MS and time of flight MS: analysis of emerging contaminants. Columbus, OH: American Chemical Society
http://simulab.ltt.com.au/5/Laboratory/PersonalStudy/psBasicsChromatography.htm
Find out how to advertise on MicroscopeMaster!
![Paper and thin Layer Chromatography By No machine-readable author provided. Dubaj~commonswiki assumed (based on copyright claims). [Public domain], via Wikimedia Commons Paper and thin Layer Chromatography By No machine-readable author provided. Dubaj~commonswiki assumed (based on copyright claims). [Public domain], via Wikimedia Commons](https://www.microscopemaster.com/images/paperandthinlayerchromatography.jpg)