Do Animal Cells have Vesicles?
** Formation and Function
Overview
Discovered in the 1940s, vesicles may be described as tiny spherical sacs enclosed by a phospholipid bilayer within eukaryotic and prokaryotic cells. There are several types that greatly vary in size (from 30nm to 1um in diameter) depending on the organism.
They are involved in a number of functions most of which revolve around the transportation of molecules.
Some of the most common types of vesicles include:
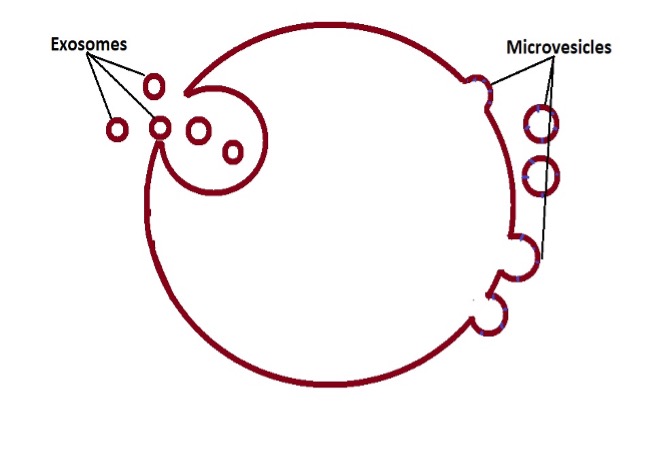
· Exosomes
· Microvesicles - Also known as microparticles
· Membrane particles
· Apoptotic vesicles/ apoptotic bodies
* Some of other other types under investigation include ectosomes and exosome-like vesicles.
* Exosomes, microvesicles, and apoptotic bodies are types of extracellular vesicles.
* Vesicles can be found in the cells of plants and animals.
Vesicles in Animal Cells
Exosomes
Exosomes are produced by all types of animal cells and measure between 30 and 150nm in diameter. Also known as intraluminal vesicles exosomes consist of a single membrane.
Because they are produced by all types of cells, exosomes can be found in various body fluids including saliva, breast milk, synovial fluid, bile, and tears, etc.
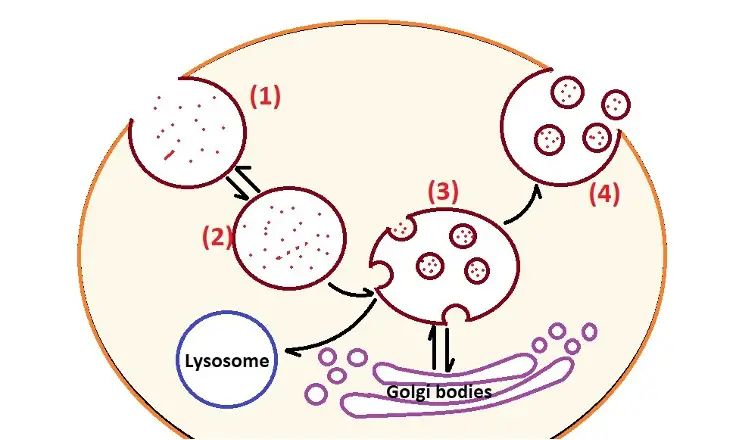
How Exosomes are formed in Animal Cells (Biogenesis)
Like some of the other types of vesicles, exosomes are of endocytic origin which means that they are formed through the endosomal route. Typically, this process started with the inward budding/invagination of the endosomal membrane to form early endosomes. The early endosomes then mature to produce multivesicular bodies which contain intraluminal vesicles.
This early phase plays an important role in the transportation of various materials into the cell. While some of the multivesicular bodies are sent to the lysosomes for degradation (along with the material they contain), some fuse with the plasma membrane so that their contents are released into the extracellular space.
Here, exosomes are some of the contents released into the extracellular space.
1. Invagination of the plasma membrane through a process known as endocytosis. This process is triggered by certain molecules/materials in the extracellular matrix.
2. Following invagination of the membrane, the vesicle separates/buds off from the membrane and moves into the cytosol. This is known as the early endosome and contains molecules/material from the extracellular matrix.
3. The early endosome matures to form the late endosome also known as the multivesicular body. This endosome interacts with the Golgi apparatus so that vesicles are exchanged between the two. As vesicles are produced from the Golgi apparatus, they come in contact with the multivesicular body causing inward budding of the membrane to form smaller bodies known as intraluminal vesicles within the late endosome/multivesicular body.
4. If the multivesicular body does not fuse with the lysosome (where its contents are degraded), it's transported to the plasma membrane where the intraluminal vesicles are released to the extracellular space as exosomes.
* Contents of the multivesicular bodies are thought to influence where it's transported to the lysosome or to the plasma membrane.
* The membrane of exosomes consist of high levels of cholesterol, ceramide, and sphingomyelin.
Though exosomes are typically formed through the classic pathway/route (described above), studies have shown that they can also be formed through the direct pathway/route. This pathway is more spontaneous and involves the release of exosomes directly from the plasma membrane of cells like T cells.
Like exosomes produced through the classic route/pathway, these exosomes are characterized by CD63 and CD81 markers and are about the same size. As such, they cannot be distinguished from exosomes released through the classic route.
Functions
Initially, exosomes were thought to be garbage/waste disposing particles of the cell. Based on new studies, however, they have been associated with a number of additional functions including cell-to-cell communication, cell maintenance, as well as tumor progression.
With regard to the immune system, some studies have shown exosomes to activate/trigger given immune responses by acting like antigen-presenting particles.
Some of the other activities associated with exosomes include:
· Promoting the formation of myelin (in nerve cells)
· Promote the growth of neurites and contribute to neuronal survival
· Contain pathogenic proteins that can promote the progression of disease in the nervous system
Microvesicles
Also known as shedding microvesicles or microparticles, microvesicles can also be found in various body/biological fluids.
Based on a number of studies, they have been shown to be larger than exosomes, measuring between 100 nm and 1.0 um in diameter. Like exosomes, microvesicles are extracellular vesicles and show some variation in shape.
Formation/Biogenesis of Microvesicles
Compared to the biogenesis of exosomes, formation of microvesicles involves outward budding of the plasma membrane. This has been shown to occur through several mechanisms.
Here, molecular cargo is first transported to the plasma membrane. This is followed by the redistribution of the membrane lipids as well as a contractile mechanism at the surface which culminated in vesicle pinching.
These include:
The ESCRT machinery (endosomal complexes required for transport) - This mechanism of microvesicle formation results in the production of Nano-sized vesicles
Non-apoptotic plasma membrane blebs - This mechanism is common in aggressive cancer cells. Here, studies have shown the blebs to continue expanding and retract at the surface of the cell where they can be released as microvesicles. In cases where they are not released, they have been shown to promote cell motility
Formation of microvesicles may be represented as follows:
Functions of Microvesicles
Microvesicles are primarily involved in the transportation of proteins, mRNA, and microRNA. Through the horizontal transfer of these molecules, they have also been associated with cellular communication. In the circulatory system, on the other hand, these vesicles are involved in several processes that include coagulation and inflammation.
For instance, based on studies involving platelets, researchers noticed that the vesicles they produce (microvesicles) may play an even bigger role in blood clotting than the platelets themselves. In peripheral blood, on the other hand, mononuclear cells have been shown to produce microvesicles during an infection.
Here, the vesicles opsonize bacteria thus promoting coagulation. Some of the other immune cells like neutrophils also produce microvesicles which promote immunity.
* Microvesicles are also involved in the horizontal transfer of cytokines and chemokine which contributes to pro-inflammatory and anti-inflammatory effects. This process also activates recipient cells to release cytokines.
* Tumor cells release vast amounts of microvesicles which promote progression of the disease.
Apoptotic Vesicles
Also known as apoptotic bodies, apoptotic vesicles are usually released by cells that are undergoing apoptosis. Compared to the other vesicles, apoptotic vesicles are larger in size, measuring between 1 and 5 um in diameter. This makes them some of the largest vesicles released by animal cells (about the same size as platelets).
Though they also have a more heterogeneous morphology, they have more or less the same density as exosomes (between 1.16 to 1.28 g/ml). Here, however, it's worth noting that there are two types of apoptotic vesicles.
The first group of these vesicles originates from the plasma membrane. These vesicles typically carry DNA and histones. Based on a number of studies, these vesicles have been shown to carry and transport these molecules to neighboring cells via the extracellular matrix or to distant cells through circulation.
The second group of apoptotic vesicles includes those that originate from the endoplasmic reticulum within the cell. Unlike those that originate from the plasma membrane, these vesicles do not contain nucleic acids and histones among other molecules.
Generally, apoptotic vesicles are formed through a budding-like mechanism. This entails three main phases that include blebbing (bulging), membrane protrusions which result in the formation of apoptopodia, and eventually pinching off of the vesicles.
Functions
As mentioned, apoptotic vesicles carry various molecules from cells that are going through apoptosis. By transporting some of these molecules (some of which are signaling molecules) to other cells, they have been shown to promote proliferation and differentiation of various cells (e.g. human endothelial progenitor cells in vitro).
Once they are released by dying cells, these vesicles can be identified and engulfed by specific cells in the body. They then release their contents and influence such activities as differentiation which can contribute to tissue regeneration.
As well, they can also activate the clearance of damaged cells.
Membrane Particles
Like exosomes, membrane particles can also be found in various body fluids (where they co-exist with exosomes). They greatly vary in size from about 50 nm to about 600 nm in diameter depending on the type of cells that produced them. As the name suggests, these vesicles originate from the plasma membrane. However, higher density is commonly observed in epithelial cells.
Wherever they are found, particularly the smaller ones, membrane vesicles are characterized by CD133+ which distinguishes them from exosomes. Like microvesicles, membrane particles are also produced through a budding-like mechanism.
Though they are not as well understood as some of the other vesicles, membrane vesicles have been associated with a range of functions including enzymatic activities in the immune system.
Return from Do Animals Cells have vesicles? to MicroscopeMaster home
References
Christopher Tricarico, James Clancy, and Crislyn D'Souza-Schorey. (2017). Biology and biogenesis of shed microvesicles.
Maojiao Li, Li Liao and Weidong Tian. (2020). Extracellular Vesicles Derived From Apoptotic Cells: An Essential Link Between Death and Regeneration.
Nina Pettersen Hessvik and Alicia Llorente. (2018). Current knowledge on exosome biogenesis and release.
Xiaona Tan et al. (2019). A Review of Plant Vacuoles: Formation, Located Proteins, and Functions.
Links
https://www.sciencedirect.com/science/article/pii/S016372581830038X
Find out how to advertise on MicroscopeMaster!