T Cells
Definition, Innate or Adaptive, Function, Vs B cells, in HIV
Definition: What are T Cells?
Originating from hematopoietic stem cells in the bone marrow, T cells, also known as T lymphocytes are cells of the adaptive immune system (also known as acquired immunity) responsible for controlling most immune responses including functions of B lymphocytes.
For the most part, this is achieved through the expression of membrane-bound molecules as well as the secretion of soluble mediators. While a majority of T cells are located in lymphoid tissues, they can be found in virtually all organs and tissues in the body (e.g. CNS, exocrine organs, mucosal sites, etc).
T cells are divided into several subsets that include:
- Helper T cells (Th)
- Cytotoxic T cells
- Natural killer cells
- Memory T cells
* "T" in T cells stands for "Thymus” - This is the organ in which the cells (T lymphocytes) mature.
Innate Vs Adaptive Immunity
As mentioned, T cells are cells of adaptive immunity. This group also includes B cells (B lymphocytes) and large granular lymphocytes known as natural killer cells.
As compared to the innate immune system, adaptive immunity is more complex given that antigens have to be processed and recognized before specific cells are released to attack the invading organism.
The innate immune system, on the other hand, provides nonspecific defense with various cells (e.g. neutrophils, eosinophils, monocytes, and basophils) and tissues/organs (e.g. mucosal linings, skin, GI tract) providing the first line of defense against invaders.
Because of the rapid response of cells of the innate immune cells, they are able to detect and partially destroy the invading organisms before cells adaptive immunity are activated and released.
While cells of adaptive immunity take more time to respond, as compared to those of innate immunity, they are able to target the invading pathogens more accurately and destroy them. Moreover, some of the cells can retain the memory of the invading pathogen which allows them to respond more effectively against them in the future.
Brief Summary of T Cells Production and Development
Essentially, T cells, like all the other blood cells, originate from the hematopoietic stem cells residing in the bone marrow. Here, the stem cells first divide to produce lymphoid and myeloid progenitor cells with the lymphoid progenitor cells differentiating further to produce T and B cells.
In the bone marrow, the T cell precursor are stimulated to migrate to the thymus gland by chemotactic agents (chemokines such as thymosin, thymotaxin, and thymopoetin, etc) released by the thymus gland. This process starts with the release of the chemokines into the blood which stimulates the migration of the T cell precursor through chemotaxis.
Within the thymus gland, the undifferentiated T cells are stimulated by chemokines and undergo changes resulting in several structural modifications. For instance, following the activation of T cell DNA by the chemokines (thymosin, thymopoetin, etc), recombinases known as RAG1 and RAG2, are produced.
These molecules then stimulate the DNA to produce different types of proteins (e.g. T cell receptor) for the different types of antigens that allow for the identification of specific antigens by the cell. By activating specific genes of T cell DNA, chemokines also stimulate the production of CD4 and CD4 proteins (cluster differentiation proteins).
Thymic cells in the thymus gland also present such molecules as MHC I and II that interact with the T cell proteins (CD4 and CD8). In the event that these proteins (CD4 and CD8 on T cell surface) identify and bind to the MHC molecules, this is known as positive selection that further contributes to the cell's development.
In a case where the molecules are not identified and bound, the T cell undergoes apoptosis since it does not function as required.
While positive selection between the MHC molecules presented by the thymic cells and CD4 and CD8 proteins of the T cells, the opposite is desired between T receptor cells (TCR) and self-antigen (peptide molecules of the body) in order to prevent autoimmune disease. In the event that the T cell receptor identifies and binds the peptides (positive selection), the T cell will undergo apoptosis.
Read more about Progenitor Cells
* In the case of undesired T cells, thymic cells produce a chemical known as Fas that binds the cells to promote apoptosis.
During T cell development, the interaction between CD4/CD8 molecules and the MHC molecules also results in a number of changes. For instance, in the event that CD4 molecules randomly interact and bind to MHCII and CD8 does not interact with MHCI molecules, genes of the T cell down-regulate the CD8 molecule while upregulating CD4 molecules.
As a result, the number of CD4 molecules on the T cell is increased while that of CD8 decreases. Cells with this characteristic are known as the T helper cells.
On the other hand, in the event that, by chance, CD8 molecules interact and bind to MHC1 molecules while CD4 molecules fail to do so with MHCII molecules, then CD4 molecules are down-regulated while CD8 molecules are upregulated to form a type of T cells known as cytotoxic cells.
* While the developmental process results in the formation of T helper cells and cytotoxic cells, these cells can also differentiate to form T regulatory cells (in the presence of CD25 and Il-2 etc) that play a role in regulating the two types of T cells.
T Cell Functions
As already mentioned, T cells play an important role in regulating immune responses. This is achieved through the expression of membrane-bound molecules as well as the secretion of soluble mediators.
Apart from their role in antibody responses and activating cells of the innate immune system, some of the T cells help suppress the immune response by controlling the duration and intensity of the immune response.
* These outcomes are achieved through the release of cytokines.
To get a better understanding of T cell functions, it's important to look at the specific functions of the different types of T lymphocytes:
Cytotoxic T cells
As previously mentioned, cytotoxic T cells are formed when CD8 molecules (glycoprotein) on their surface identify and bind to the peptides presented by MHCI molecules. For this reason, these cells are often referred to as CD8+ cytotoxic T cells (or simply CD8+ T cells).
As such, they are characterized by CD8, a dimeric co-receptor consisting of a single chain of CD8α and a single chain of CD8β.
In the body, cytotoxic T cells play an important role in defending the body against such pathogens as bacteria and viruses that invade body cells as well as destroying malignant and infected cells. Once they identify specific antigens, the cells are activated and respond in several ways to destroy infected and malignant cells.
Once they are activated by the presence of certain antigens (e.g. from malignant and infected cells), T cells respond by secreting such cytokines as Tumor Necrosis Aactor-alpha and Interferon-gamma that have anti-tumor and anti-microbial properties and ultimately contribute to apoptosis of infected and malignant cells.
Activation of T cells may result in the secretion and release of cytotoxic granules that consist of perforin and granzymes. To destroy the infected or malignant cells, perforin first binds to the target cells and causes pores to form on the cell membrane.
Once the pores are formed on the membrane, granzymes (serine proteases) are able to easily enter into the cell and cleave cell proteins. This activity interferes with the production of viral proteins or other processes within malignant cells and ultimately results in apoptosis of the cell.
* Serine proteases are a type of enzyme capable of cleaving peptide bonds.
* To avoid damaging healthy neighboring cells, the destruction of infected and malignant cells by cytotoxic granules occurs in a serial fashion. Here, the cell releases granules and destroys an infected cell before moving on to new targets. This process is often referred to as serial killing.
Apart from producing and releasing cytokines and cytotoxic granules, cytotoxic T cells also respond by expressing FasL for Fas/FasL interaction. Here, FasL (Fas ligand) expressed by the T cells interact and bind to the Fas on the surface of the target cell (infected or malignant).
As a result of this interaction, Fas molecules trimerize on the surface of the infected/malignant cell thus causing aggregation of signaling molecules. In turn, these molecules activate caspase cascade which causes apoptosis of the cell.
* Because of the mechanism through which they destroy infected and malignant cells, cytotoxic cells can also cause organ rejection during organ transplant given that the new cells are identified as foreign and thus targeted.
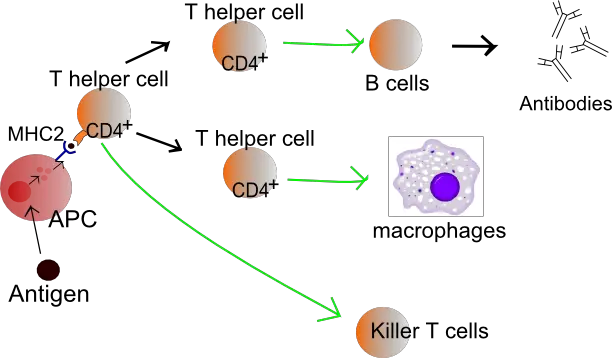
Helper T Cells
In some books, helper T cells have been described as the most important immune cells of the adaptive immune system, not only because of their role in influencing the maturation and activities of B cells, macrophages, and memory cells, but are also involved in the activation of cytotoxic T cells.
Therefore, while they do not directly target and destroy invading micro-organisms and affected cells, they play an important role in regulating the activity of different types of immune cells in response to invading organisms and infected/malignant cells.
As compared to cytotoxic T cells, helper T cells express the CD4 proteins and are therefore also referred to as CD4+ helper cells. As already mentioned, helper T cells play an important role in regulating activities of a number of other immune cells.
As such, they are described as effector cells. To achieve this, however, helper T cells also have to be activated.
Typically, this occurs when they are exposed to peptide antigens by MHCII molecules on the surface of antigen-presenting cells. In peripheral lymphoid tissue, the activation of naive helper T cell can result in the production of several types of specialized cells.
These include:
- T-helper 1 (Th1)
- T-helper 2 (Th2)
- T-helper 17 (Th17)
- Follicular helper T cell (Tfh)
- Induced T-regulatory cells (iTreg)
- Regulatory type I cells (TrI)
While there are several subsets of helper T cells, this section will largely focus on the classical T-helper cells (Th1 and Th2).
T Helper I
Following activation, naive helper T cells can differentiate into ThI cells which contribute to immunity by secreting IFN-y (interferon-y) and TNF-α (tumor necrosis factor-α). Typically, T-helper I cells are activated by antigen-presenting cells harboring invading microbes.
When dendritic cells are infected by certain bacteria (e.g. bacteria that cause tuberculosis), they migrate to such peripheral lymphoid organs as the spleen and lymph nodes where they activate Th1 cells.
Once they are activated, Th1 cells activate macrophages (by secreting IFN-y) to destroy the invading microbes. Here, dendritic cells act as antigen-presenting cells and thus play an important role in processing antigen material and presenting it to the helper T cells.
This is particularly important given that it allows macrophages to destroy the microorganisms (e.g. bacterial) located in their own phagosomes.
* At the site of infection, dendritic cells can produce a number of molecules including MHCII proteins and cytokines (e.g. IL-12) which stimulates the differentiation of naive T cells into Th1 effector cells to continue activating macrophages in order to destroy invading microbes.
* By stimulating dendritic cells to secrete more costimulatory proteins, Th1 cells also activate cytotoxic T cells and also contribute to the destruction of virus-infected cells by these cells.
T Helper 2 Cells
Whereas T-helper 1 cells secrete molecules that contribute to the destruction of microbes that reside within given cells (e.g. bacteria that invade the phagosome of macrophages), T-helper 2 cells contribute to immune responses against extracellular pathogens (e.g. helminths).
Moreover, they are also involved in tissue repair. They have also been associated with a number of allergic disorders and inflammatory diseases.
Once activated, T-helper 2 cells release a number of lymphokines that help destroy or expel the invading organisms from the body. By releasing interleukin 4 (IL-4), Th2 activates the synthesis of IgE antibodies by B cells.
The antibodies then bind to a number of immune cells including mast cells and eosinophils and stimulate them to secrete and release local mediators which in turn promote such outcomes as diarrhea, coughing and sneezing to expel invading pathogens/parasites from the body.
Some of the other interleukins secreted by Th2 include:
- IL-4
- IL-5
- IL-13
- IL-10
Functions of the other types of T helper cells:
· T-helper 17 (Th17) - As T helper cells that secrete IL-17A, IL-17F, IL-21, and IL-22, T helper 17 cells are primarily involved in defensive mechanisms against extracellular bacterial infections. They have also been shown to play a role in a number of autoimmune diseases.
· Follicular helper T cells - Follicular helper T cell (Tfh) is a subset of T helper cells found in secondary lymphoid organs like the spleen, tonsils and lymph nodes. Here, they form sites known as germinal centers where B cells proliferate, mature and produce different types of antibodies.
In addition, they are sites in which B cells differentiate into plasma cells and memory B cells involved in antibody production.
· Induced T-regulatory cells - Induced regulatory T cells (iTreg) are a type of T cell that develop from peripheral naive conventional T cells in the thymus. In the body, iTreg cells play a role in enhancing immunological tolerance.
· Regulatory type I cells - Tr1 cells are a subset of CD4+ T cells that like Induced T-regulatory cells, also play a role in promoting immune tolerance. They are also involved in regulating inflammation mediated by effector T cells as well as antigen-presenting cells.
CD4+ and HIV
While helper T cells play an important role in the destruction of invading microbes that reside within cells, the HIV virus (Human Immunodeficiency Virus) has been shown to target and attack these cells.
As a result, the number of helper T cells (CD4+) is significantly reduced which in turn affects the production of antibodies by B cells and cytotoxic T cells that destroy infected cells and the microbes they harbor. In the event that the number of CD4 drops to below 200, the patient is diagnosed with AIDs.
Memory T Cells
Memory T cells may be described as antigen-specific T cells that remain in the body for a long time (they can remain in the body for the entire duration of one’s' lifetime) even when an infection has been eliminated. They are either CD4+ or virus-specific CD8+ and include effector memory T cells, central memory cells, and effector memory RA.
Maintenance of these cells in the body is achieved through two main mechanisms that include:
Antigen-independent homeostatic proliferation (constant turnover) - This is a slower mechanism that maintains mature T cells in the periphery.
Antigen-driven lymphocyte proliferation - In mice, antigen-driven turn-over has been shown to occur about once every 35 days (Memory T cells multiply and differentiate rapidly when they are re-stimulated by antigen).
Once activated, memory T cells respond as effector cells that directly destroy invading pathogens. For instance, following an infection by such intracellular pathogens as viruses, memory T cells are reactivated and respond by expressing cytolytic effector functions.
Here, then, they destroy the invading intracellular pathogens through such cytolytic proteins as perforin, Fas, and Granzyme. These proteins successfully enter the cell and affect the normal development of pathogens thus stopping their development and proliferation.
Memory cells are also capable of producing such proinflammatory cytokines and chemokines as CCL3, CCL5, and IFNy that activate and recruit other cells of the immune system to action.
As compared to several other cells of the immune system, memory T cells have several advantages that enable them to quickly respond to the invading pathogens. However, these properties also cause them to react negatively to allografts during organ/tissue transplants.
One of the biggest advantages of these cells is the fact that they respond faster and at a greater magnitude compared to naive T cells. This is largely due to the fact that following antigenic re-stimulation, memory T cells are capable of generating a significant number of effector T cells that produce cytokines or respond through cytolytic activity against the invading pathogen. Their populations persist for a long period of time.
Because they are re-stimulated by antigen and MHC, they continue persisting in the body and can respond within hours. Lastly, memory T cells circulate through the secondary lymphoid tissues as well as peripheral non-lymphoid tissues and thus directly respond to infections in these sites within a short period of time.
Natural Killer Cells
Natural killer cells are a type of cytotoxic lymphocytes and thus have granules that contain such proteins as perforins. Once they are activated by interferons and cytokines following an infection (e.g. virus-infected cells), natural killer cells secrete perforins that form pores on the surface of the infected cells allowing for proteases (granzymes) to enter and cause apoptosis.
This allows for the infection to be contained before other cells of the immune system migrate and clear the infection.
T Cells Vs B Cells
While B cells and T cells (both are lymphocytes) belong to the adaptive immune system, differences can be identified in how they are produced as well as how they respond to invading pathogens.
Whereas T cells migrate to and mature in the thymus gland, B cells mature in the bone marrow (both T and B cells and produced in the bone marrow). With regards to immune response, B cells provide humoral immunity while T cells provide cell-mediated immunity. Therefore, while T cells migrate to the infected site in response to the invading organism, B cells do not.
In their response, T cells may also act against transplants or malignant cells in addition to invading pathogens. However, B cells only respond to invading pathogens. One of the other major differences between the two is that while B cells have a short lifespan, T cells have a long lifespan and can remain in the body for the duration of one’s lifetime.
Some of the other differences between the two include:
- B cells have surface antigens while T cells do not
- B cells produce antibodies while T cells produce lymphokines
- T cells recognize pathogen antigens on the surface of infected cells while B cells recognize them on the surface of the pathogens themselves
Return to learning about Immunology
Return from learning about T Cells to MicroscopeMaster home
References
Erika Wissinger. (2016). CD8+ T Cells. Imperial College London, UK.
Fadi G. Lakkis and Mohamed H. Sayegh. (2003). Memory T Cells: A Hurdle to Immunologic Tolerance.
Grégoire Lauvau and Saïdi M’Homa Soudja. (2016). Mechanisms of Memory T Cell Activation and Effective Immunity. ncbi.
Lakna Panawala. (2017). Difference Between T Cells and B Cells. ResearchGate.
Rishi Vishal Luckheeram, Rui Zhou, Asha Devi Verma, and Bing Xia. (2012). CD4+T Cells: Differentiation and Functions. ResearchGate.
Links
https://www.immunology.org/public-information/bitesized-immunology/cells/t-follicular-helper-cells
https://www.ncbi.nlm.nih.gov/books/NBK279396/
Find out how to advertise on MicroscopeMaster!