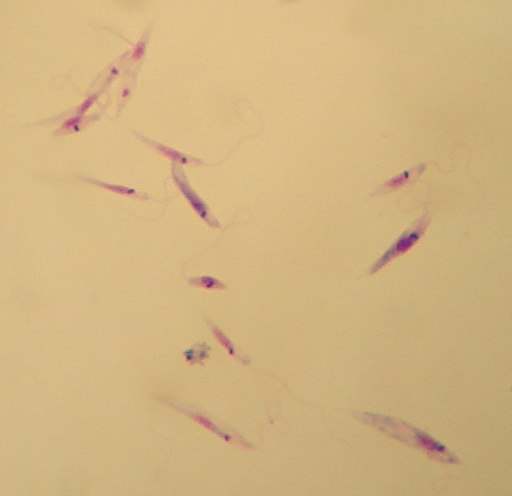
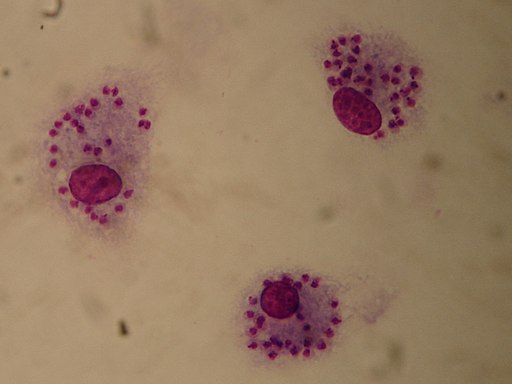
Leishmania
Definition, Classification, Life Cycle and Infection
What is Leishmania?
Leishmania is a genus of the family Trypanosomatidae and consists of parasitic protozoa. As a member of the family Trypanosomatidae, this species are obligate parasites that require a host for their survival. Compared to other members of this family, however, they are heteroxenous and thus require two hosts to complete their life cycle.
In vertebrates, Leishmania species are responsible for a spectrum of diseases (e.g. visceral diseases and mucocutaneous diseases, etc) whose symptoms vary depending on the species of the parasite and host vulnerability.
According to studies, over 50 species of Leishmania described so far are spread across all continents (tropic and sub-tropic regions) except the Antarctica with about 20 species being responsible for human infections.
Some of the most common species of the genus Leishmania include:
- L donovani
- L tropica
- L arabica
- L aethiopica
- L orientalis
- L senegalensis
- L major
- L amazonensis
Classification of Leishmania
Kingdom: Protista
Subkingdom: Protozoa
Phylum: Sarcomastigophora - Phylum of the kingdom Protista that consists of unicellular or colonial (colonies) that are characterized by the presence of pseudopodia or flagella
Class: Zoomastigophora - This class consists of free-living or parasitic protozoa that use one or more flagella for locomotion (a few species may lack these structures)
Order: Kinetoplastida - Made up of flagellated protists characterized by a large mass of DNA known as kinetoplast
Family: Trypanosomatidae - As compared to other members of the order Kinetoplastida, members of the family Trypanosomatidae have a single flagellum. Whereas some of the organisms in the family are free-living while others exist as parasites, they all present a Kinetoplast being members of the order Kinetoplastida
Genus : Leishmania
Subgenus: Leishmania, Sauroleishmania, Mundinia (also known as enriettii complex) and Viannia
* Leishmania species are also divided into old world species and new world species. Whereas old world species include those from Asia, Africa, and Europe, new world species include those occurring in America.
Viannia Vs Leishmania Subgenus
Species of the sub-genera Viannia and Leishmania are closely related and also well understood as compared to the other sub-genus. However, they also have a number of characteristics that are unique to each group. For instance, members of the sub-genera Viannia have been shown to undergo prolonged development in the cuticular intima of the hindgut of the vector.
Here, the paramastigotes and promastigotes are characterized by a rounded appearance and are often attached to the cuticular intima by their flagella. As they develop, they gradually move to the mid-gut and ultimately the foregut before they are transferred to the next host.
On the other hand, the subgenus Leishmania is characterized by parasites that develop in the mid-gut and foregut of the vector.
Life Cycle
As already mentioned, members of the genus Leishmania are heteroxenous and thus require two hosts (vertebrate and invertebrate hosts) to complete their life cycle. Whereas the vector acts as the intermediate hosts, human beings and over 60 animal species serve as the reservoir hosts of the parasite.
Vector
Currently, sand flies are the only known vectors of the parasite. Found in inter-tropical and temperate regions across the globe, there are over 600 Leishmania species divided into five main genera.
Only those in the genus Phlebotomus (referred to as phlebotomine sand flies) and Lutzomyia have been shown to be vectors of human leishmaniasis. Whereas species in the genus Phlebotomus are vectors of the parasite in the Old World, those of the genus Lutzomyia act as vectors in the New World.
During the life cycle of Leishmania species, the vector (female sand flies) acquires the parasite when they feed on the infected blood of the host. Like female Anopheles, female sand flies (that act as vectors for Leishmania species) also depend on mammal blood for egg development.
Unlike the malaria parasites found in peripheral circulation (within red cells), amastigote forms of the parasite consumed by the vector as it feeds on blood are found in the phagolysosomes of macrophages located in the skin.
* Apart from the skin, amastigote forms of the parasite are also present in such organs as the spleen and liver. As such, they are not easily available to the vector
During feeding, sand flies use their mouthparts to cut through the skin which in turn creates small wounds on the skin. By agitating these wounds, the vectors are able to feed on blood flowing from superficial capillaries in the skin which contains macrophages in which the parasites reside.
Following ingestion by the fly, the amastigote starts differentiating into the early stages of procyclic promastigotes in the mid-gut. These forms multiply and increase in numbers (through longitudinal binary fission) while attached to the interior wall of the mid-gut to avoid being expelled along with other excrements. This is made possible by Lipophosphoglycan molecules found on the surface of the parasite.
* Lipophosphoglycan is a branched molecule complex consisting of polypeptides and polysaccharides and particularly important during species-specific interactions.
Here, differentiation is characterized by morphological changes of the parasite from spherical (spherical shape of the amastigotes) into an ovoid and elongated shape. Being the motile forms, the promastigotes also have a well-pronounced flagellum used for motility. Using this flagellum, the promastigote forms are able to migrate from the mid-gut to the foregut.
Using the flagellum, the parasite is also able to attach itself to gut microvilli of the vector as well as stomodeal valve. Apart from motility and attachment, studies have also suggested that the flagellum has sensory functions.
Apart from blood, the sandflies also feed on sugar as an energy source. Using the flagellum as a sensory organ, it's suggested that the parasite is able to successfully navigate the sugar gradient in order to migrate to the foregut before it's transmitted to the next host.
This phase of cell division and multiplication is then followed by another phase of differentiation where the parasite differentiates into nectomonad promastigotes. These cells then migrate to the anterior part of the mid-gut where they give rise to leptomonad promastigotes that ultimately produce metacyclic promastigotes.
Identification of these cell types is largely based on the size (length and width) of their bodies. For instance, whereas nectomonad promastigote are about 12um in length, leptomonad promastigote are characterized by a body length that is between 6.5 and 11.5um as well as a flagellum that is longer than the cell body.
Also, the metacyclic promastigote (haptomonad promastigote) which is the last cell type produced in the vector has a body cell that is less than 8um long and a flagellum that is longer than the cell body.
Apart from the different developmental sequences between them, the cells are also found in different locations in the vector. For instance, whereas the nectomonad promastigotes can be found in the peritrophic matrix before it starts breaking down, the leptomonad promastigotes are found in the foregut having escaped the peritrophic matrix.
It's this form that then differentiates to give rise to the infective form of the parasite (metacyclic promastigote). Despite the differences in size, the different forms of the parasite in the vector have similar basic cell architecture consisting of a kinetoplast as well as an anterior flagellum.
* Multiplication of the parasite within the vector can give rise to as many as 35,000 parasites from an average of 3,200 ingested during feeding (in 1.6 um of a single blood meal).
Vertebrate Infection - Life Cycle
Following a week of division and multiplication, the infective forms of the parasite (metacyclic promastigotes) block the anterior gut and pharynx of the vector and therefore have to be regurgitated. As the sand fly takes a blood meal, the infective forms are deposited into the wound (wound caused by the feeding behavior of the vector).
In response to this damage, macrophages are recruited to the infected site. Here, the infective forms of the parasite infect these immune cells. However, given that these forms (metacyclic promastigotes) are highly motile, studies have shown that it's possible for the interaction between the parasite and macrophages to interact in sites other than the wounded site of the skin.
Following the engulfment of the parasite, the flagellum (flagellum of the parasite) has been shown to continue moving which causes damage to the plasma membrane of the macrophage. In addition, the poor integrity of the cell caused by the flagellum contributes to lysosomal exocytosis thereby allowing the parasite to successfully invade and inhabit the cell.
Once the parasite successfully inhabits the macrophage, it starts to differentiate giving rise to form with a more spherical cell body and short flagellum. This significant reduction in size is suggested to reduce the surface area over which the cell is exposed to the environmental conditions that can cause damage to the parasite.
* During this stage, the flagellum changes from the 9+2 axoneme which is long and motile to the 9+0 axoneme arrangement that is short and non-motile.
* Given that the flagellum is not completely lost when the parasite inhabits the macrophage, it's assumed that it has sensory functions to play within these cells.
Within the macrophage, the parasite starts differentiating to form the amastigote which is an obligate intracellular, non-motile form. Mitotic division allows the parasite to proliferate and thus continue invading new phagocytic cells as they increase in numbers.
This continues until the macrophages are ingested by the vector when they feed on blood. Once the parasites are acquired by the sand fly, they undergo several stages of development and the cycle continues.
Reservoir Hosts
Apart from human beings, a good number of animals (dogs, cattle, etc) also act as hosts for the parasite. While a majority of these animals may not exhibit any symptoms, they are beneficial to the parasite given that they allow the life cycle to continue. This has been shown to contribute to zoonotic leishmaniases where the infection can be transmitted from wild and domestic animals to human beings.
In anthroponotic leishmaniases, on the other hand, human beings act as the reservoir hosts and are directly infected once they are bitten by an infected vector (sandfly).
Some of the other animals that act reservoir hosts for the parasite include:
- Rock Hyraxes
- Bats
- Mongoose
- Wolves
- Armadillos
Adaptations of Leishmania Parasite
In order to successfully thrive in the host, Leishmania parasites use a number of mechanisms that are primarily aimed at evading activities of immune cells.
Once they enter the body of the vertebrate (human beings and other animals), the infective forms of the parasite (metacyclic promastigotes) prevent insertion of C5-C9 membrane attack complex which prevents them from being lysed.
The membrane attack complex (MAC) present on the surface of pathogens act as effector proteins that activate complement system of the host. As a result, they initiate immune responses that ultimately result in cell lysis and thus cell death. By preventing the insertion of C5-C9 membrane attack complex, the parasite is able to avoid lyses.
And so, using such surface molecules as LPG (lipophosphoglycan ) and GIPL (glycosylinositol phospholipid), the parasites are able to attach to and invade responding macrophages and continue dividing within these cells.
To subvert inflammatory responses, the parasites can recruit molecules that suppress the cytokine signaling family and thus prevent the activation of immune cells that would otherwise destroy them.
This is achieved in a number of ways including the activation of de-ubiquitinating enzyme A20 of the host by the parasite as well as recruitment of such molecules as the SOCS-1 and SOCS-3 that suppress cytokine signals.
To survive the harsh conditions within the macrophages in which they reside, Leishmania parasites have developed a number of mechanisms. By preventing the fusion of phagosome and lysosome, the parasite delays the maturation of endosomes which contribute to the functions of the lysosome.
Some of the other mechanisms through which Leishmania parasite avoid actions of the immune system include:
- Affecting normal antigen presentation
- Interfere with normal cell signaling
- Modify T cell responses
* In some hosts, the parasites are phagocytized and destroyed by macrophages in the body of the host. This not only prevents the infection from spreading, but also protects the host from re-infection in the future.
Disease Mechanism
Whereas the parasite is easily eradicated in some hosts, the infection may spread to various organs in some hosts. Following inoculation of the parasite in the skin, a cutaneous infection may occur.
Depending on the individual, this may be followed by a chronic localized infection that can disfigure the affected part of the skin. This may be characterized by the development of a Leishmanian skin ulcer that can progressively worsen if not attended to.
Among immunocompromised individuals, the infection can spread rapidly affecting different types of organs and interfering with their normal functions.
Forms of Leismaniasis
There are three main forms of Leishmaniasis.
They include:
· Visceral leishmaniasis - Also known as Kala-azar, visceral leishmaniasis is characterized by bouts of fever, liver and spleen enlargement, anemia as well as weight loss. It is particularly common in parts of Brazil, Asia and Eastern parts of Africa. Untreated, visceral leishmaniasis has been shown to be fatal in most cases.
· Cutaneous leishmaniasis - is the most common form of Leishmaniasis. It is characterized by skin lesions that tend to leave life-long scars. If left untreated, the disease can cause disabilities by affecting given tissues and their functions. Cutaneous leishmaniasis is common in parts of Asia, the Americas and the Mediterranean basin while new cases are being reported in Brazil, Iran and Algeria among other countries.
· Mucocutaneous leishmaniasis - is the type of Leishmaniasis responsible for damage caused at the mucous membranes of the mouth and throat etc. This may prove fatal in some cases and is particularly common in such countries like Brazil, Peru, and Bolivia.
Treatment and Prevention

For the most part, prevention involves avoiding sandfly bites. Given that sandflies tend to be more active between dusk and dawn, avoiding outdoor activities during this period of time may prevent one from getting bit and infected.
Using insect repellents for exposed skin and sleeping under the mosquito net can help significantly reduce the chance of getting bit.
Some of the other methods used to prevent the infection include:
· Vector control - Using insecticides to control sand flies can help reduce transmission of the disease
· Control reservoir hosts - Depending on the situation, controlling reservoir hosts (animals) can reduce zoonotic infections
With regards to treatment, no medicines capable of getting rid of the parasites are currently available. However, among those with a normal functioning immune system, lesions caused by the parasite tend to self-resolve within a few weeks.
In some cases, however, this may require close monitoring by health care providers. Depending on the patient and the level of infection, some of the options available include repeated cryotherapy using liquid nitrogen and local heat therapy.
Physicians have also experimented with a number of medicines including:
- Amphotericin B
- Antimonial compounds
- Oral ketoconazole such as Kuric and Xolegel
Return to learning about Trypanosoma, and Acidocalcisomes
Return to Parasites under the Microscope
Return to Parasitology main page
Return from Leishmania to MicroscopeMaster home
References
Bereket Alemayehu and Mihiretu Alemayehu. (2017). Leishmaniasis: A Review on Parasite, Vector and Reservoir Host. Health Science Journal.
Gaurav Gupta, Steve Oghumu, and Abhay R. Satoska. (2014). Mechanisms of Immune Evasion in Leishmaniasis. ncbi.
Jack Sunter1 and Keith Gull. (2017). Shape, form, function and Leishmania pathogenicity: from textbook descriptions to biological understanding. nbci.
Ralph Lainson and Jeffrey J. Shaw. (1992). A brief history of the genus Leishmania (Protozoa: Kinetoplastida) in the Américas with particular reference to Amazonian Brazil .
Stephan Klatt ,Larry Simpson,Dmitri A. Maslov,Zoltán Konthur. (2019). Leishmania tarentolae: Taxonomic classification and its application as a promising biotechnological expression host.
Links
https://www.asmscience.org/content/book/10.1128/9781555816728.chap134
Find out how to advertise on MicroscopeMaster!