What do Plasma Cells do?
Structure and Function
Overview: What are Plasma Cells?
Also known as plasma B cells, plasma cells are terminally differentiated B lymphocytes. While they originate from activated B cells in the spleen and lymph nodes (secondary lymphoid organs) etc., some plasma cells migrate to the bone marrow where they may persist for an extended period of time.
Here, it's suggested that they interact with the stromal cells that surround the sinusoidal endothelial cells which facilitates the production and release of antibodies into the blood stream.
Apart from a short lifespan, though some plasma cells have been shown to survive for several months to years, plasma cells are also characterized by their ability to produce large amounts of antibodies (proteins produced in response to antigens).
Apart from their immune functions, plasma cells have also been associated with several regulatory functions that include:
- Regulating hematopoiesis
- Regulating neuro-inflammation
Structure of Plasma Cells
While plasma cells have some resemblance to lymphocytes, they have some differences that make it possible to distinguish them. Generally, plasma cells are relatively larger in size (ranging from 14 to 20 um) and contain more cytoplasm which stains deep blue during histochemistry.
They also have an eccentrically placed nucleus. Not centrally placed, but rather occupies one side of the cell in the cytoplasm characterized by thick chromatin.
Unlike the chromatin found in other lymphocytes, microscopic studies have revealed a unique pattern in plasma B cells resembling an art-wheel or clockwork pattern. Here, however, the nucleus may vary between cells from uninucleate (contain a single nuclei) to multinucleate (contain five or more nuclei) - Uninucleated plasma cells are the most common.
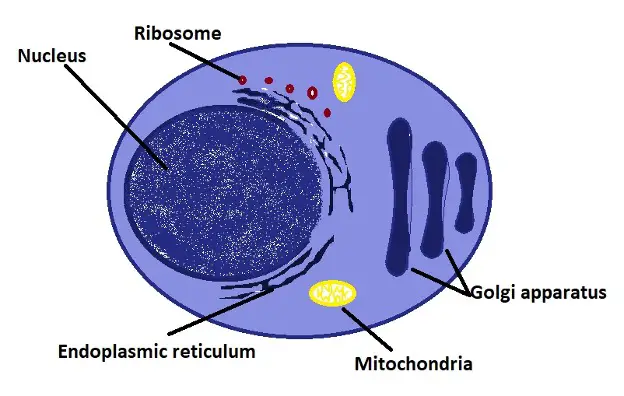
Plasma B cells also contain an abundance of rough ER (rough endoplasmic reticulum) and Golgi apparatus that allows them to effectively carry out their functions namely producing immunoglobulins (antibodies).
Like other cells, they also contain a number of other important organelles including mitochondria, ribosomes, and lysosomes among others.
Depending on the cells, some have been shown to contain Russell bodies (cytoplasmic inclusions) associated with secretion.
Diagrammatic representation of a plasma cell:
Functions
As mentioned, plasma cells have a number of functions in the body.
These include:
Production of antibodies
Plasma cells are primarily involved in the production of antibody molecules that bind to specific antigens so that they can be destroyed.
Like memory B cells, plasma cells also originate from activated B cells (B lymphocytes produced in the red bone marrow). When they are released from the bone marrow, these B cells migrate to the lymph nodes and spleen where they reside.
Using immunoglobulin on their surface, they are able to identify given antigens which results in the antigen being internalized into the cell through a process known as endocytosis. The antigen is then broken down allowing the cell to express MHC complex (class II) on its surface.
When helper T cells identify the antigen bound to the major histocompatibility complex on the surface of B cells, they release the co-stimulation molecules required for B cell proliferation and differentiation.
Here, the differentiation process is especially important as it results in the production of memory B cells and plasma cells (effector B cells).
* Unlike memory B cells, plasma cells are short-lived. However, it's worth noting that memory B cells will also rapidly transform, through heightened proliferation and differentiation, into plasma cells when they encounter an antigen for a second time
* The antibodies of plasma cells closely resemble receptors (immunoglobulin) located on the surface of B cells. However, unlike those receptors which contain a transmembrane domain that links them to the plasma membrane of B cells; plasma cell antibodies can be released so that they float in circulation.
Once they are formed and settle in the bone marrow, spleen, and lymph nodes, plasma cells do not migrate from one tissue to another as is the case with some of the other immune cells. For this reason, their localization plays an important role in the production and release of antibodies into circulation.
In the spleen and lymph nodes, where they are commonly found, plasma cells are located among the reticular sinusoidal cells within the red pulp and medullary cords characterized by high vasculature.
There are several types of antibodies produced by plasma cells.
These include:
· IgM - Consist of four constant domains for each of the heavy chains
· IgG - Make up the majority of all antibodies and are characterized by heavy chains associated with a variable domain and three constant domains that are identical to each other
· IgA - Characterized by three constant domains for each of the heavy chains
* A single plasma cell can release thousands of antibodies that enter circulation. When memory B cells recognize given antigens, they rapidly proliferate and differentiate into plasma cells that produce numerous antibodies. This allows for the invading pathogen to be eliminated quickly known as the secondary immune response.
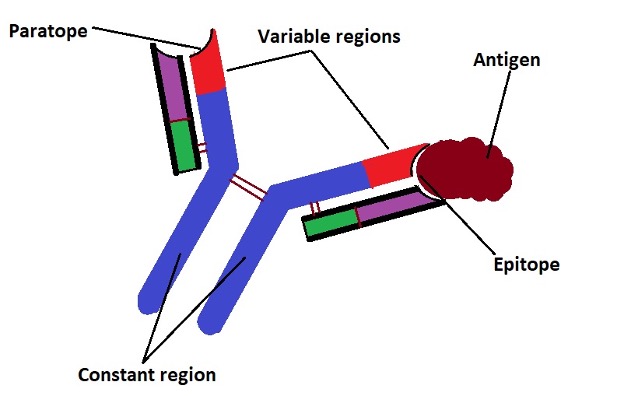
In circulation, antibodies will interact with specific antigens to neutralize them. As mentioned, the different types of antibodies produced by plasma cells consist of a constant region (domain) as well as a variable region.
While the constant region, as the name suggests, consists of the same peptide chains, peptide chains of the variable region can change thus contributing to antibody specificity. Memory B cells are capable of "remembering" different types of antigens.
In the presence of given antigens, they proliferate and differentiate to produce plasma cells capable of producing antibodies for specific antigens. When the antibody comes in contact with the antigen, the part of the antigen known as the epitope is bound to the antibody at the paratope (antigen binding site which recognizes the antigen).
The following is a diagrammatic representation of this interaction:
As the antibody and antigen interact, molecular studies have shown that the ionic and hydrophobic forces contribute to this reaction. Here, water is expelled as the bond between the two increases.
The binding is reinforced by van der Waals force . Some of the other bonds that may be involved include hydrogen or electrostatic bonds.
By binding to the active sites of antigens and pathogens (which coats the pathogens), the pathogens/antigens are neutralized as they are rendered ineffective. For instance, by binding to the active sites of a virus, antibodies prevent the viral particle from adhering and gaining entry into a cell.
Following this reaction between the antibody and antigen, the antigen/pathogen may be eliminated in several ways that include:
Filtration - As the antibody and antigen combine to form a complex, a process known as agglutination, they can be filtered by the spleen (along with old and worn out red blood cells) so that they can be removed from the body along with feces and the urine.
Opsonization - Refers to the process through which given molecules are modified in a manner that allows phagocyte receptors to have a stronger affinity for them. In the body, IgM and IgM first bind to specific antigens resulting in the formation of sites onto which complement proteins bind.
This complex enhances the optimization process where the invading pathogen is cleared by macrophages. This is also an important process that allows antigens to be presented to T cell for increased immune response.
Complement activation (also known as complement cascade or complement system) - Here, antibodies and phagocytic cells are involved in eliminating the invading pathogen from the body. This mechanism involves a number of important steps that start with the antibody binding an antigen located on the surface of a pathogen.
This activated the complement protein (C1) which in turn cleaves the complement components C2 and C4 resulting in the production of C4b2b. This convertase transforms the complement component C3 in C3a and C3b into the convertase C5. The convertase then converts complement component C5 into C5a and C5b.
Together with complement components C6 and C7, C5b forms a complex which again forms a complex with C8 and C9 to produce a membrane attach complex that is responsible for lysing the pathogen.
Ultimately, this process breaks down the membrane thus compromising the integrity of the cell.
The Role of Plasma Cells in Hematopoiesis
In mice and human beings, plasma cells have been shown to play a role in hematopoiesis (production of blood cells from hematopoietic stem cells located in the bone marrow). As mentioned, some of the plasma cells, namely long-term plasma cells, are located in the bone marrow where they have been shown to reside for an extended period of time.
Here, about 80 percent of these cells are in contact with the stromal cells that maintain hematopoietic stem cells and the hematopoiesis process. While bone marrow stromal cells also play an important role in the survival of plasma cells located in this niche, the depletion of plasma cells in mice was shown to result in reduced myelopoiesis.
Based on these results, researchers have concluded that bone marrow cells have an impact on hematopoiesis.
While plasma cells can influence hematopoiesis, this is, for the most part, age-dependent. Unlike old plasma cells, young plasma cells do not seem to have a significant impact on myelopoiesis.
For old plasma cells, promoting myelopoiesis generally involves the adoption of inflammatory gene signature by the plasma cells which is magnified by toll-like receptor stimulation. On the other hand, plasma cells tend to suppress B lymphopoiesis.
Here, the interaction between neoplasm produced by plasma cells, stromal cells, and multiple myeloma cells results in higher production of chemokine ligand 3 (C-C Motif Chemokine Ligand 3), chemokine ligand 4 (C-C Motif Chemokine Ligand 4), and TGFB1 (transforming growth factor-beta one) which have been associated with suppressed B lymphopoiesis.
Gut Homeostasis
Plasma cells (especially IgA plasma cells) can be found in the gut where a variety of microbes reside. Here, these cells have also been shown to play an important homeostatic role. In the gut, resident plasma cells produce secretory IgA which has two main functions including the exclusion of certain bacteria, that react with the antibody IgA, as well as promoting an immune response.
In mice, plasma cells are also capable of promoting the production of regulatory T cells (Treg) by producing retinoic acid and transforming growth factor beta. In doing so, plasma cells are indirectly involved in maintaining intestinal immune tolerance.
Like the skin, the intestinal tract consists of a wide range of microbes, both beneficial and pathogenic.
Given that a good number of microbes have beneficial functions (e.g. bacteria involved in digestion), cells of the immune system have to be prevented from destroying these cells. The regulatory T cells achieve this by controlling immune responses both innate and adaptive.
* As mentioned, plasma cells in the gut can react with some of the bacteria in the gut. Here, IgA antibodies produced by these cells bind to the antigens of these bacteria so that they can be eliminated.
This contributes to the removal of pathogenic bacteria as the cells (gut plasma cells) promote immunological tolerance of the good bacteria.
Regulate Neuro-Inflammation
Based on a number of studies, involving the use of mice subjects, plasma cells have been shown to be capable of regulating neuroinflammation. In conditions like autoimmune encephalitis, plasma cells can produce interleukin 10 (IL10) which has a suppressive effect on autoimmune encephalomyelitis-induced pathology.
Essentially, interleukin 10 is a cytokine characterized by anti-inflammatory properties. As such, it regulates immune responses to various pathogens which minimize the damage that would otherwise be caused to tissue. In the absence of this cytokine, heightened immunopathology would occur as immune cells respond to various infections.
In the process, this may also result in autoimmune conditions. By producing the cytokine, plasma cells play an important role in suppressing neuro-inflammation thus preventing further damage to neural tissue.
* Plasma cells that regulate neuro-inflammation (by producing interleukin 10) have been shown to originate from the small intestine.
* While studies involving mice have shown plasma cells to regulate neuro-inflammation, this is not necessarily the case with some autoimmune disorders affecting the nervous system. In the case of neuromyelitis optica, for instance, the production of auto-antibodies that target aquaporin are thought to contribute to the condition.
Return to learning about B Cells
Return from What do Plasma Cells do? to MicroscopeMaster home
References
Caitlin Bohannon et al. (2016). Long-lived antigen-induced IgM plasma cells demonstrate somatic mutations and contribute to long-term protection.
Heather A Minges. (2005). Plasma Cells.
Hunter C. Allen and Poonam Sharma. (2020). Histology, Plasma Cells.
Oliver J. Harrison and Fiona M. Powrie. (2013). Regulatory T Cells and Immune Tolerance in the Intestine.
Peter D. Pioli. (2019). Plasma Cells, the Next Generation: Beyond Antibody Secretion.
Links
https://www.labce.com/spg448425_plasma_cell.aspx
https://opentextbc.ca/biology/chapter/23-3-antibodies/
Find out how to advertise on MicroscopeMaster!