Fibroblasts
Histology, Wound Healing, and Macrophages/Myofibroblasts
Definition: What are Fibroblasts?
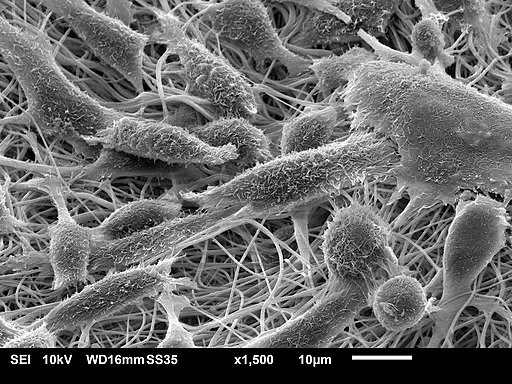
Commonly found in interstitial spaces/compartments, fibroblasts are spindle-shaped cells (flat and elongated) primarily involved in the production of extracellular matrix and collagen.
Being versatile tissue-resident cells, they are also involved in several other functions including angiogenesis, sending local signals and tissue repair among, etc.
Along with a number of other cells (e.g. bone and cartilage cells), fibroblasts make up a family of cells known as connective-tissue cells. As such, they are found throughout the body where they are involved in supporting other tissue by providing structural integrity within connective tissue.
* Fibroblasts have also been defined as stromal cells that do not express markers for specific mesenchymal lineage.
Brief Overview of Fibroblast Production
Like the other cells making up the connective tissues, fibroblasts have been shown to originate from the embryonic mesoderm tissue. Based on a variety of studies, however, fibroblasts are suggested to have several different origins during different stages of development.
Traditionally, it has been accepted that fibroblasts originate from mesenchymal cells that in turn originate from epithelial-mesenchymal transition (EMT). A few days after postnatal life, these fibroblasts start proliferating thus increasing the overall number of these cells.
Apart from the rapid proliferation of fibroblasts, this increase in the overall number of cells has also been suggested to be a result of the EMT pathway. Apart from the mesenchymal origin, some of the studies hold that fibroblasts originate from hematopoietic stem cells.
In adults, some of the studies have shown that over time, fibroblasts divide from the resident fibroblasts (as well as being the products of the EMT pathway). Other studies suggest them to originate from such progenitor cells as pericytes and mesenchymal cells located in the bone marrow.
Fibroblasts origin may, therefore, be summarized as follows:
- Resident fibroblast cells
- EMT pathway
- Hematopoietic stem cell
- Hematopoietic progenitor cells
- Other progenitor cells (E.g. pericytes)
- Subpopulation of monocytes
Fibroblast Functions
Although fibroblasts are versatile, they are primarily involved in the production of extracellular matrix (ECM) components of connective tissues that provide structural and biochemical support.
Some of the main components of ECM produced by fibroblasts include:
Structural Proteins
In the body, fibroblasts are involved in the production of various proteins (protein fibers and connector proteins) that contribute to the tissue structure.
Depending on the tissue and part of the body, these proteins (structural proteins) have varying properties and serve different functions.
A good example of this is the triple-stranded helical tertiary protein structure of collagen type-1 that contributes to the overall rigidity of fibrils. This is because of the fact that the protein structure provides tensile strength which prevents overstretching.
As compared to collagen type I, elastin proteins allow for stretching without breaking. This allows the tissue in which they are found (e.g. the skin) to stretch as needed.
Adhesive Proteins
In addition to the structural proteins, fibroblasts also produce such adhesive proteins as laminin and fibronectin that serve to connect cells to the extracellular matrix. Here, these adhesive proteins not only contribute to the general architecture of extracellular matrices, but also influence cellular phenotype by attaching the surfaces of different types of cells.
By attaching to different types of cells, the adhesion proteins may influence motility and differentiation.
Why is Cell Adhesion important?
Ground Substance
Fibroblasts are also responsible for the production of ground substances that occupy the space between cells and fibrillar elements. Being a cell-free medium (that consists of a hydrated gel); the ground substance is involved in nutrient flow as well as cell migration.
* Here, it's worth noting that in the body, fibroblast cells produce smaller molecules that ultimately form finished collagen, elastin, and fibronectin, etc once they are released. Therefore, fibroblasts do not produce these proteins in finished forms.
By looking at the different components of the extracellular matrix mentioned above, it becomes clear that fibroblasts are involved in different functions through the production of ECM.
In human skin, for instance, components of ECM are divided into fiber-forming molecules (e.g. collage, fibrin, and elastin, etc), nonfiber forming molecules as well as proteins that do not have structural functions.
Whereas the fiber-forming molecules are involved in providing the structure through the creation of complex rigid proteins, nonfiber-forming molecules are involved in the creation of charged, dynamic, and osmotically active spaces.
Some of the other functions of extracellular matrix include:
- Acting as filler between the cells
- Retain a given water level
- Help maintain homeostatic balance
- Influence tissue morphology
- Act as storage for such molecules as cellular growth factors
Apart from producing important (and maintaining) components of the extracellular matrix, fibroblasts are also responsible for their reabsorption. Whereas fibroblasts control the maturation of collagen by producing an enzyme known as Lox (lysyl oxidase), these cells also produce such degrading enzymes as metalloproteinases that regulate/breakdown collagen.
Through extracellular matrix regulation, fibroblasts maintain the appropriate levels of extracellular matrix which in turn contributes to the proper functions of this complex network.
Role of Fibroblasts in Wound Healing
Essentially, wound healing occurs through three main stages that include the inflammatory phase, the proliferative phase, as well as the regeneration phase.
Following damage to the capillaries, a blood clot starts forming in order to prevent further bleeding. This being the inflammatory phase, different types of cells are recruited to the site of injury under the influence of chemokines released by platelets. Among these cells are macrophages and fibroblasts that arrive at the site through a process known as chemotaxis.
While the first phase of wound healing (the inflammatory phase) stimulates the recruitment of fibroblasts to the site of injury, the second phase (proliferative phase) is particularly important in this function in that it promotes the proliferation of fibroblasts.
During this stage of wound healing, new blood vessels start forming which allows for nutrients to be transported to the site. Consequently, this allows fibroblasts recruited to the site to proliferate and thus increase in numbers.
Following the activation of fibroblasts, in granulation tissue (connective tissue and tiny blood vessels formed during wound healing), they acquire Alpha Smooth Muscle Actin (α-SM) allowing them to be transformed into myofibroblasts.
The transformation of fibroblasts to myofibroblasts is an important process during wound healing given that the new cells are involved in the production and release of extracellular matrix components that replace the provisional matrix and thus contribute to the wound healing process.
These newly formed myofibroblasts produce high amounts of cytoplasmic microfilaments, also known as actin-rich stress fibers as well as other stress fibers that contribute to their contraction.
This, in addition to the presence of gap junctions and desmosomes which join cells, help in the reduction of the area of granulation tissue by pulling collagen fibrils present in extracellular matrix towards the cell body.
During this phase of wound healing (remodeling phase), synthesis of the extracellular matrix also reduces with myofibroblasts ultimately undergoing apoptosis as the wound closes.
* While fibroblasts contribute to the wound healing process, a scar is left at the injury site. According to a number of studies, however, it's possible for cutaneous wounds to heal without scarring at the fetal stage. This has been attributed to the fact that as compared to adult fibroblasts, fetal fibroblasts have a number of beneficial characteristics.
Compared to adult fibroblasts, fetal fibroblasts have been shown to not only migrate at a much faster rate, but also capable of being more efficient when it comes to organizing extracellular matrix. Moreover, they have been shown to consistently produce hyalonuric acid which in turn contributes to rapid migration, repair process as well as collagen cross-linking.
Role of Fibroblasts in Inflammation and Immune Response
In the event of an injury, fibroblasts, like a number of other cells, respond to various cytokines and migrate to the site of injury where they contribute to wound healing.
Fibroblasts have been shown to be capable of producing inflammatory cytokines that stimulate given immune cells to respond.
In addition to cytokines, fibroblasts can also produce the following components:
- Antimicrobial peptides
- Chemokines
- Toll-like receptors
- Growth factors
Receptors
Like some of the cells of the immune system, fibroblasts have been shown to be capable of expressing Toll-like receptors (TLRs) against various microorganisms. Currently, about 10 types of these receptors have been identified on the surface of skin fibroblasts.
Once they identify the presence of pathogen-associated molecular pattern molecules (PAMPs), fibroblasts respond by producing cytokines and a number of antimicrobial molecules thereby contributing to the immune response against the pathogen (e.g. Gram-negative bacteria and viruses).
* Some of the TLRs expressed by fibroblasts include TLR4 (in the presence of certain bacteria and corneal cells etc) as well as TLR3 in response to viral molecules.
Antimicrobial agents
Apart from receptors that sense the presence of various microorganisms, fibroblasts have also been shown to produce a number of antimicrobial agents against invading pathogens.
In the presence of invading microorganisms (such as mycobacteria species), fibroblasts have been shown to produce such antimicrobial agents as cathelicidins and defensins.
In the presence of Chlamydia spp, intracellular bacteria, gingival fibroblasts produce hBD-2. Through these activities, fibroblasts contribute to immune responses in different parts of the body such as the gums and cornea.
Cytokines
In addition to responding to various cytokines, fibroblasts also produce a number of cytokines that allow them to communicate with other cells.
Some of the cytokines produced by fibroblasts include:
- GFβ1
- IL-1β
- IL-33
- IL-8
- IL-6
- INFy
Through these factors, fibroblasts activate the migration of resident immune cells (e.g. macrophages) against the invading microorganisms.
While fibroblast are capable of stimulating macrophages, macrophages (and particularly M2 macrophages), immune cells that are also involved in wound healing have also been shown to influence the production and activities of fibroblasts.
Here, macrophages achieve this by producing a variety of factors:
Growth Factors
In such fibroblasts as those found in human gums (gingival fibroblasts), studies have shown them to be capable of producing growth factors. For instance, in the gums, these cells produce GM-CSF while fibroblasts located in the cornea produce GMC-CSF.
By producing growth factors, fibroblasts stimulate immune responses against various microbial infections thus contributing to the immune responses. Apart from immune responses, these factors have also been shown to contribute to the proliferation of immune cells (e.g. macrophages, eosinophils, etc) in addition to promoting cancer pathogenesis.
 Fibroblast Growth Factor by Nevit Dilmen / CC BY-SA (http://creativecommons.org/licenses/by-sa/3.0/)
Fibroblast Growth Factor by Nevit Dilmen / CC BY-SA (http://creativecommons.org/licenses/by-sa/3.0/)
The Role of Fibroblasts in Disease Development
While fibroblasts have a number of important functions in the body, they have also been associated with a number of diseases.
Fibrosis
Under normal circumstances, myofibroblasts (that develop from fibroblasts) undergo programmed cell death once a wound heals. However, in some cases, these cells persist which allows scarring to continue. Because they do not undergo apoptosis, myofibroblasts continue producing excess matrix and consequently causing excess tissue contraction.
As compared to hypertrophic scars, keloid scars continue to grow and expand over time past the edges of the wound. Here, fibroblasts located in these scars also continue proliferating with the surrounding fibroblasts also being activated.
In some cases, this excessive scarring can affect the normal functioning of the affected organs/tissue. For instance, in the case of pulmonary fibrosis, the lungs can become increasingly thickened making it difficult for an individual to breathe.
Cancer
Apart from their role in fibrosis, fibroblasts have also been associated with the development of cancerous cells. For this reason, stiffness of the matrix is used as one of the indicators of the risk of malignancy.
While fibroblastic cells themselves have been shown to be capable of causing certain types of cancer, the interaction between non-cancerous fibroblasts and cancer cells may also affect the pathogenesis of these cells.
According to studies, non-cancerous fibroblasts around tumorous cells play an important role in angiogenesis, which ensures that these cells are continually supplied.
As well, by remodeling connective tissue around the tumor, fibroblasts promote the release of cancerous cells into the vascular system thus contributing to metastasis (development of secondary malignant growths).
Histology of Fibroblasts
For histological purposes, fibroblasts from given tissues have to be prepared appropriately. Depending on the tissue of interest, a number of methods (e.g. shave biopsies, core biopsies and punch biopsies, etc) can be used to obtain the tissue for preparation.
For example, to obtain skin fibroblasts, shave biopsy can be used. Here, a sharp blade is used to remove a thin piece of skin to obtain a tissue sample.
During cell preparation, the unwanted parts (e.g. fat and loose fascia) are first removed followed by the digestion phase (to digest adhering primary cells). This step is then followed by cell isolation before cell culture. The incubated culture is then observed under the phase-contrast microscope to study cell characteristics.
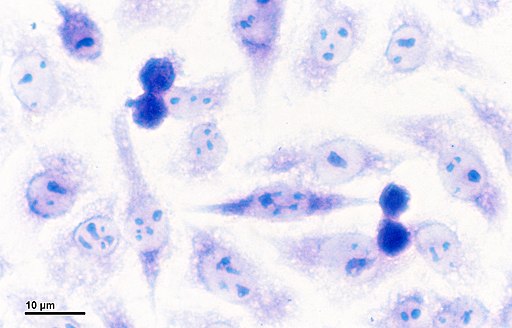
* When viewed under the microscope, fibroblasts portray an elongated spindle shape with cytoplasmic projections. When viewed under an electronic microscope, it's possible to identify rough endoplasmic reticulum as well as Golgi apparatus.
* Under the standard light microscope, only the nucleus is clearly visible near the collagen fibers.
Return from learning about Fibroblasts to MicroscopeMaster home
References
Chang. Y, Li. H, and Guo Z. (2014). Mesenchymal Stem Cell-Like Properties in Fibroblasts. Cellular Physiology and Biochemistry.
Lauren E. Tracy, Raquel A. Minasian, and E.J. Caterson. (2016). Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. ncbi.
Luis Antonio Bautista-Hernández, José Luis Gómez-Olivares, Beatriz Buentello-Volante, and Victor Manuel Bautista-de Lucio. (2017). Fibroblasts: The Unknown Sentinels Eliciting Immune Responses Against Microorganisms.
Mary K. Dick, Julia H. Miao, Faten Limaiem. (2019). Histology, Fibroblast.
Ryan T. Kendall and Carol A. Feghali-Bostwick. (2014). Fibroblasts in fibrosis: novel roles and mediators. ncbi.
Sue Porter. (2007). The role of the fibroblast in wound contraction and healing.
Links
https://www.researchgate.net/publication/215638163_Heterogenecity_of_fibroblasts
Find out how to advertise on MicroscopeMaster!