What is the Function of B Cells?
Development, Maturity & Vs T Cells
Definition of B Cells
Also known as B lymphocytes, B cells are professional antigen-presenting cells that make up between 5 and 15 percent of the total lymphocytes in circulation.
In response to antigens in their surroundings, B cells express major histocompatibility complex class II as well as co-stimulatory molecules (e.g. IL-2, IL-4, IL-6, etc) that not only activate T cells but also influence their own differentiation into and plasma cells (antibody producing B-cells).
Along with T lymphocytes, B lymphocytes make up the second line of defense (adaptive immunity) that protects the body against specific invading/foreign pathogens and the substances that they produce.
Based on the stage of development, B cells can be divided into three main groups that include:
- Naive B cells
- Memory B cells
- Plasma cells
* The "B" in B cells stands for bursa of Fabricius; a lymphoid organ located behind the proctodic region of the cloaca in birds. In birds, this is the site production and development of B lymphocytes.
Development
B cells are first produced in the fetal liver during the early stages of development. Based on studies involving mice, researchers noticed that during the production of these B cells, CXCL10 and CXCL12 (chemokine ligands) first attract CXCR3 and CXCR4-expressing pHSCs among other progenitors and influence their movement to the developing liver from the embryonic blood.
Here, they migrate into the stromal cells where they supply IL-7 among other molecules to the hematopoietic and B-lymphopoietic progenitors thereby activating the development of early B cells.
In adults, however, B cells are produced through the differentiation of hematopoietic stem cells located in the bone marrow (a primary lymphoid organ). From the primary lymphoid organ, they migrate to the secondary lymphoid organs where they undergo further development.
In the bone marrow (in the endosteal niche), the hematopoietic stem cells (HSCs), which are pluripotent in nature receive signals and thus undergo differentiation to produce progenitors known as lymphoid progenitor cells.
Unlike hematopoietic stem cells, these cells are multipotent and only capable of giving rise to a few types of cells. Once they receive the appropriate signal from the stromal cells in the bone marrow, these cells undergo division and differentiate to give rise to the earliest B cells.
Once the synthesis of RAG-1 and RAG-2 and Terminal deoxynucleotidyl transferase (TdT) synthesis in CD34+ lymphoid progenitors are activated by cytokines, these multipotent cells undergo D-J joining on the H chain chromosome which transforms them into pro B cells.
Further development is then characterized by the V segment joining the D-JH. When they express the membrane Mu (u) chains with surrogate light chains, these cells again change to become pre-B cells. Therefore, the pro/pre BI cells are some of the earliest types of B cells found in the bone marrow.
Generally, these cells are characterized by CD19 and CD117 expression as well as the arrangement of IgH chain loci in a D-J configuration.
During the development of B cells within the bone marrow, it's worth noting that antigens are not involved. Therefore, these cells are not yet exposed to antigens at this point - this phase is largely characterized by VDJ recombination.
The following are some of the characteristics of the different forms of B cells within the bone marrow:
Pro B Cell
- Earliest for of B cell in the bone marrow
- At this stage, the cell is yet to undergo DVJ combination
- Lacks the B cell receptor and thus has nothing on its surface
- During the early stage of the Pro B cell, it undergoes recombination of heavy chain D and J this is followed by recombination of D to the DJ chain that formed early on
* The processes involved in this stage result in the formation of the heavy chain on the cell surface. This transforms the Pro B cell into a Pre B cell.
Recombination-activating genes 1 and 2 (RAG 1 and 2) are presented during the Pro B cell phase given that they are involved in the formation of the heavy chain.
Pre B Cell
As mentioned, the development of the heavy chain on the surface of the pro-cell transforms it into the Pre B cell. Therefore, one of the differences between the two is the fact that the pre B cell has a heavy chain on its surface. However, this chain does not fold properly without a light chain.
Because the light chain has not yet been formed, the heavy chain is first paired with two chains known as surrogate light chains (SL) before the light chain is formed. The two surrogate chains include VpreB and Lambda 5 (λ5). Along with the heavy chain, the two chains form the Pre BCR
Although the light chain is not yet formed, the surrogate chain (along with the heavy chain which form the cell receptor with the surrogate chains) allow the cell to receive a signal - This is important as that signal reception allows the cell to survive and proliferate.
Cell proliferation allows the cell to increase in number with the heavy chain being reproduced. This also allows for variation of the light chain that will be formed following this proliferation.
If the heavy chain proves to be a good chain, the RAG 1 and 2 are turned back on and activate the formation of the light chain (Kappa light chain) – For the immature B cell, the light chain pair with the u chain to form the monomeric IgM. In association with Igα/Igβ at the surface of the cell, the IgM forms the B cell receptor.
* Once the light chain has formed, the Pre B cell becomes known as an immature B cell.
* As good number of cells do not form the heavy chain and therefore die through apoptosis. The other cells that form good heavy chains continue developing and eventually become immature B cells.
Once the immature B cell is produced, it continues to receive signals (from IL-7) through the receptors which prevent it from dying. This also activates cell proliferation so that they increase in numbers. Moreover, the light chain is also tested to determine whether it's functional.
In the event that it fails to develop properly, the cell will die and not develop further. Cells that pass this test leave the bone marrow and are transported to the secondary lymphoid organs where they mature further. Before being released, they have to undergo another test (to test self-reactivity) in order to determine whether they bind self-antigens.
Here, the cells that bind self-antigens have to be destroyed given that they would otherwise cause autoimmunity where they attack the body of the host.
Maturity
As mentioned, the first phase of B cell development takes place within the bone marrow. Here, the stromal cells in the bone marrow are responsible for sending the signals that influence the development of B cells during the antigen-independent phase (development within the bone marrow).
Ultimately, the entire process within the bone marrow results in the production of an immature B cell (immature transitional B-cells) that consists of a B cell receptor (immunoglobulin) commonly known as IgM (Immunoglobulin M). This is the first and largest antibody found in vertebrates. From the bone marrow, the C cell migrates to the secondary lymphoid organ.
From the bloodstream, successful immature B cells migrate to the spleen (a secondary lymphoid organ). Here, the immature B cell, which is at this point characterized by IgM and IgG on its surface, is commonly referred to as transitional type I B cell.
Along with T cells and follicular dendritic cells, the B cell forms the primary follicle (white pulp). This is an important region that exposes the B cells to various self cells as well as proteins in circulation to test whether they respond to these cells and molecules strongly.
In the event that they respond strongly, they are activated to become T3 B cells (anergic cells) that eventually die off. By responding to self sells and other molecules in circulation, these cells indicate that they are likely to cause auto-reactions and are therefore highly likely to be responsible for autoimmunity.
For this reason, they have to be inactivated so that they can eventually die off. It's worth noting that B cells that do not receive signals during the development process cannot continue developing. B cells that survive are allowed to move into the follicle where they transition to T2 B cells.
As such, they are able to respond to antigen and are commonly referred to as naive follicular B cells. These cells are capable of migrating to the other lymphoid tissues where they can identify antigens.
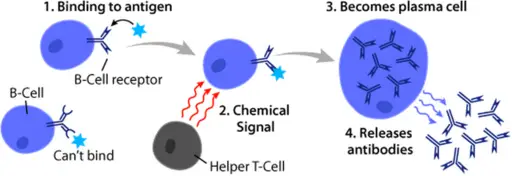
When the mature naive cells bind to an antigen (through the B-cell receptor), they become activated and start differentiating to form plasma cells. Here, the B cells start by binding to the antigen (or pathogen-associated molecular patterns) via the B cell receptor. This has two important consequences namely, proliferation of the B cells as well as internalization of the protein antigens.
These antigens are digested before being presented to the T cells through the major histocompatibility complex class II (MCHII - an antigen-presenting molecule common among professional antigen-presenting cells).
The interaction between these cells results in the activation of the B cells through CD-40. T cells activate the B cells through the CD40L (ligand) which is expressed on their surface.
CD40 (costimulatory protein) is also present on the surface of the B cell. In addition, T cells also produce cytokines that act as signals that stimulate the differentiation of B cells to produce plasma cells.
* Plasma cells are antibody-producing cells.
Antibodies
Also known as immunoglobulin ("immuno" refers to the immune system while "globulin" refers to the protein), (Ig), antibodies are specific proteins produced by plasma cells in response to pathogenic antigens.
Being highly specific proteins, antibodies are able to identify and bind specific antigens so that they, and associated pathogens, can then be destroyed.
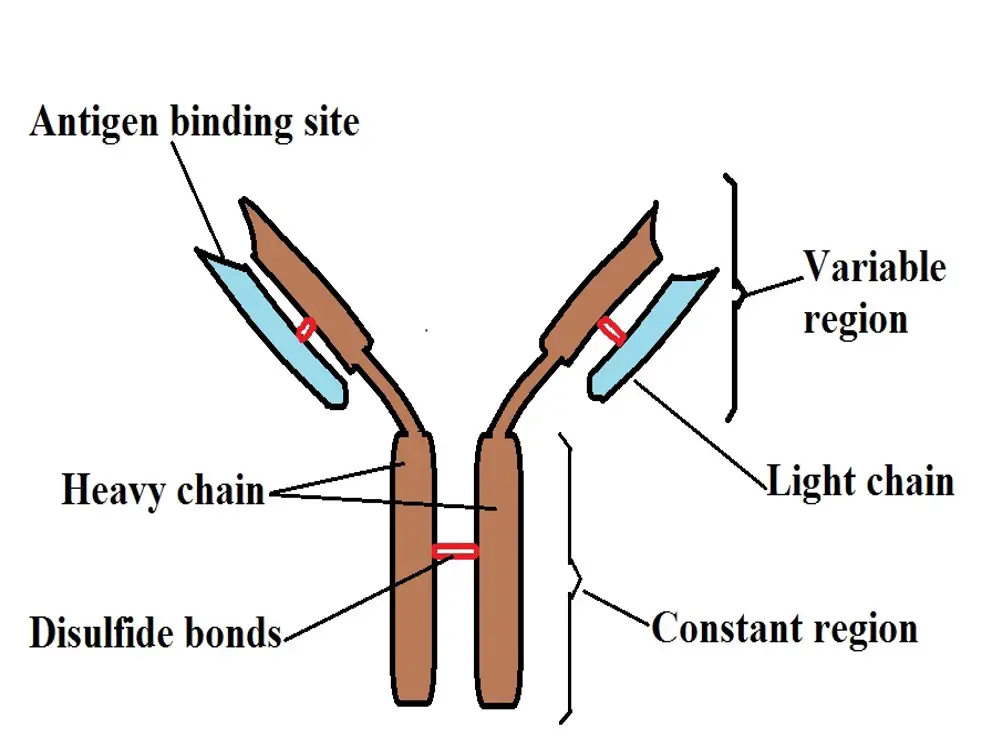
Although there are different types of antibodies, the immunoglobulin consists of four polypeptides that are joined through disulfide bridges (disulfide bonds) which are themselves covalent bonds.
This results in a Y-shaped structure that consists of a constant and variable region.
The following is a diagrammatic representation of an antibody:
* Generally, the light chains (in blue) and smaller compared to the heavy chains (in brown). The red bands in the diagram represent the disulfide bridges/bonds. In addition to linking the heavy chains, they also join the light chains to the heavy chains.
* The variable region of the antibody consists of a specific sequence of amino acids allowing the antibody to bind to specific antigens.
Given that the sequence of amino acids varies between different antibodies, this region is commonly referred to as the variable region.
As compared to the variable region, the constant region does not change for antibodies within the same group. However, it varies between the different types of antibodies (IgD, IgA, and IgG, etc).
As such, it's used to determine the different types of antibodies - The constant region is the part of the antibody that binds to the cell membrane.
While antibodies are produced by plasma cells in response to specific antigens, some of the antibodies can be found in circulation without being attached to the immune cell. However, they can also be found attached to the cell membrane.
Regardless, they are able to locate and bind to the specific antigen resulting in the formation of an antibody-antigen complex.
Depending on the antigen, this complex may have different consequences that include:
Inactivation of the pathogen - In an instance where antibodies bind to such pathogen as viruses, studies have shown antibodies to prevent the pathogen from binding onto the receptor located on the host cell (target cell of the virus). As a result, the pathogen is inactivated and prevented from proliferating.
Marking/labeling the pathogen/infected cell - The antibody/antigen complex also allows the invading pathogen or the infected cell to be labeled. This is particularly important as it allows white blood cells (e.g. phagocytes) to identify and destroy these cells.
Aggregation - The formation of an antibody/antigen complex can also result in aggregation to form an insoluble complex that ultimately inactivates the invading pathogen.
In some cases, this causes several invading pathogens, along with the antibodies, to aggregate and form larger insoluble complexes. This also inactivates the pathogen and prevents them from dividing and proliferating.
As mentioned, there are different types of antibodies.
These include:
IgG - Immunoglobulin G (IgG) is the most abundant antibody in the body with 65-70 percent of them being found in the blood plasma. It is a monomer and thus exists as a single Y-shaped antibody.
Although it's produced during the primary immune response, studies have shown that IgG is particularly abundant during the secondary immune response (when an individual is exposed to the antigen for the second or third time, etc).
Following binding to the antigen, this antigen can cause neutralization, opsonization, or precipitation of the invading pathogen. This antibody can be transferred from the mother to the fetus through the placenta.
IgA - Unlike Immunoglobulin G which exists as a monomer, Immunoglobulin A is a dimmer which means that two antibody monomers are joined together. They can be found in different parts of the body including the skin, the gastrointestinal tract (mucosal lining), as well as the saliva, etc.
In addition, IgA antibodies can also be found in the milk of the mother and can therefore be transferred to the baby during breastfeeding. They bind to the antigens of invading pathogens in the regions mentioned above which in turn allows for these invading organisms to be targeted and destroyed.
IgM - Immunoglobulin M is the type of antibody that has been shown to exist as either a monomer or in a pentameric form. Whereas the monomers exist singly, pentameric forms consist of five (5) monomers joined together.
As already mentioned, IgM is the first type of antibody to be produced in the body. It's mostly produced during the primary immune response. They are commonly found in the extracellular matrix in different parts of the body where they are involved in opsonization and agglutination.
IgE - Immunoglobulin E also exists as monomers and is commonly found in the mucosa of the respiratory tract, some lymphatic tissues as well as some of the urogenital structures, etc. This antibody is involved in allergic reactions as they are produced in response allergens.
In addition to allergic reactions, IgE also binds to antigens of various parasites including helminths which allow them to be destroyed.
IgD - Like some of the other Immunoglobulin, IgD is also a monomer. They exist as B cell receptors and thus play an important role in B cell activation.
B Cells Vs T Cells
Together with natural killer cells, B cells, and T cells are both lymphocytes and can be found in the lymph, various tissues as well as blood. While B cells and T cells have a number of similarities (e.g. they both originate from the bone marrow), they have several differences with regard to their development and functions.
Essentially, B cells and T cells originate from the stem cells located in the bone marrow where they share the same morphology early on in their development. However, whereas B lymphocytes continue to develop in the bone marrow during the antigen-independent phase, T cells migrate to the thymus (another primary lymphoid organ) where they develop and mature.
Following the development of both cells, B cell receptors are formed on the surface of B cells and constitute antibodies. As well, T cell receptors are formed on the surface of T cells. Unlike B cell receptors, these receptors are different from the membrane antibodies associated with plasma cells.
B cells are also less abundant compared to T cells, only making up about 20 percent of the total blood lymphocytes. With regard to function, B cells are primarily involved in humoral immunity, which is an antibody-mediated immunity, while T cells are involved in a cell-mediated type of immunity.
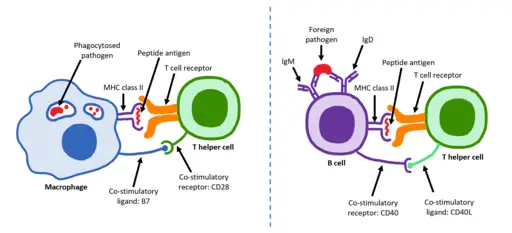
Regardless of these differences, the two types of lymphocytes work together to protect the body against invading pathogens. As already mentioned, B cells have B cell receptors on their surface. These receptors bind with antigens on the surface of various invading pathogens and activate the internalization of the pathogen through a process known as receptor-mediated endocytosis.
Some molecules of the pathogen are then placed on the surface of the cell, on the major histocompatibility complex class II (the molecules are presented to T cells through the MHCII). This allows the helper T cells to bind to the MHCII and release lymphokines that activate the proliferation and produce plasmablasts that give rise to plasma cells.
In response to the initial antigen, these plasma cells produce antibodies that identify and target the pathogen. Here, it becomes possible to see how the two types of cells work together.
Some of the other differences between B and T cells include:
· B cells bind to various foreign antigens as well as foreign material in circulation while T cells bind to antigens that are associated with self-antigens
· T cells do not bind directly to the antigen (but rather to the infected cells) while B cells directly bind to the antigens
· B cells give rise to memory cells and plasma cells while T cells produce helper T cells, cytotoxic T cells, as well as memory cells
· T cells tend to last longer than B cells in the body
Return to Lymphocytes main page
Return from What is the Function of B Cells? to MicroscopeMaster home
References
James T. Cassidy et al. (2011). Textbook of Pediatric Rheumatology.
Joshua A. Boyce, Fred Finkelman, and William T. Shearer. (2013). B-cell biology and development. Mechanisms of allergic diseases.
Lakna Panawala. (2017). Difference Between T Cells and B Cells.
Robert Brink. B Lymphocytes. Immunology and Immunopathogology.
Silke Häusser-Kinzel and Martin S. Weber. (2019). The Role of B Cells and Antibodies in Multiple Sclerosis, Neuromyelitis Optica, and Related Disorders.
Links
https://www.intechopen.com/books/lymphocytes/understanding-b-lymphocyte-development-a-long-way-to-go
https://mainebiotechnology.com/b-cell-differentiation/
Find out how to advertise on MicroscopeMaster!